
Future Prospects and Growth Opportunities in the Generic Drugs Market
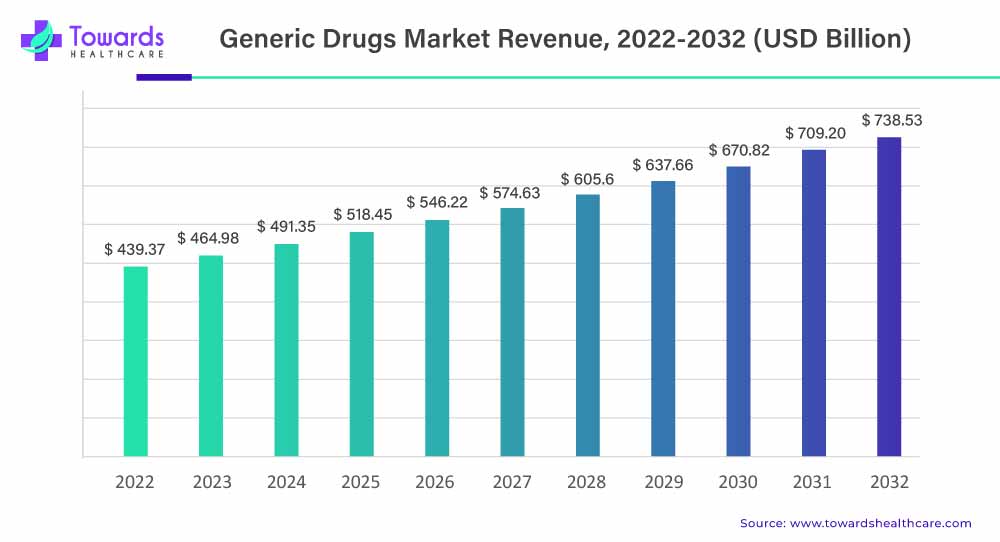
The global generic drugs market is expected to experience significant growth, increasing from USD 439.37 billion in 2022 to approximately USD 738.53 billion by 2032, reflecting a compound annual growth rate (CAGR) of 5.3% from 2023 to 2032. This growth is driven by the rising number of generic drug approvals and increased investments in the sector, which are helping to expand access to affordable medications worldwide.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5053
The Impact of Funding on the Growth of the Generic Drugs Market
Increased funding is playing a pivotal role in the development and growth of the generic drugs market. It supports crucial research and development (R&D) efforts that help bring new generic drugs to market. For example, in 2021 and 2022, the FDA allocated $20 million each year to fund research and programs that focus on advancing generic drug science. This funding is part of the FDA’s initiative to make affordable, high-quality generic drugs more accessible to patients in the United States.
Generic drug manufacturers use this financial support to invest in areas like developing new drug formulations, improving manufacturing processes, and conducting studies to ensure that their products are just as effective as their brand-name counterparts. With more funding, these companies can expand their R&D capabilities, ensuring that more generic drugs meet the high standards required for market approval.
The generic drugs industry operates under strict regulations to guarantee the safety, quality, and effectiveness of medications. Funding helps manufacturers meet these regulatory standards by supporting clinical trials, obtaining certifications, and ensuring their production facilities meet the necessary requirements. With adequate financial backing, companies can navigate the complex regulatory process, helping them secure approvals for their generic drugs.
Funding is also critical for building and expanding manufacturing capabilities. Investments in advanced facilities and equipment that comply with Good Manufacturing Practices (GMP) standards enable manufacturers to produce high-quality products on a larger scale. This allows them to meet growing market demand while maintaining product integrity.
In addition to improving production, funding helps generic drug companies expand their reach. With financial support, they can establish partnerships with wholesalers, distributors, and healthcare providers to make generic drugs more widely available. Funding also facilitates marketing and market research efforts, helping to raise awareness about the benefits of using generic drugs.
As the market continues to grow, funding plays a key role in helping manufacturers expand internationally. It enables them to explore new markets, form partnerships with local distributors, and navigate different regulatory environments. With the right financial backing, generic drug manufacturers can tailor their products to meet regional needs and successfully enter new markets.
Regional Overview of the Generic Drugs Market
The generic drugs market in North America, especially in the United States, is a vital and rapidly growing sector. The U.S. plays a leading role in the global generic drugs market, contributing a significant share to overall market revenue. In 2021 alone, 6.4 billion prescriptions were filled in the U.S., with 91% of those being for generic or biosimilar drugs. These more affordable alternatives to brand-name medications are crucial in making healthcare more accessible. The widespread use of generics resulted in impressive cost savings, totaling $373 billion across various groups, including patients, consumers, employers, and taxpayers. This clearly shows the important role generic drugs play in making healthcare more affordable in the U.S.
In fact, generic pharmaceutical companies manufacture the medications for 9 out of every 10 prescriptions filled in the country. This underlines just how essential generics are to providing affordable healthcare options to millions of people. The U.S. healthcare system actively supports the use of generic drugs through policies and initiatives aimed at making them more accessible. One such initiative is the U.S. Food and Drug Administration’s (FDA) Generic Drug User Fee Amendments (GDUFA), which helps speed up the approval process for generics. These efforts are designed to encourage competition, lower drug prices, and increase the availability of generics.
In 2021, the top 10 generic drugs made a remarkable contribution to cost savings, amounting to $110 billion. These generics, as affordable alternatives to branded medications, have played a significant role in reducing the overall cost of healthcare. The ten most popular generics alone saved $66 billion, showcasing their impact on healthcare affordability.
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/generic-drugs-market
Access exclusive insight now @ https://www.towardshealthcare.com/price/5053
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare




