Companion Diagnostics Market Insights and Innovation
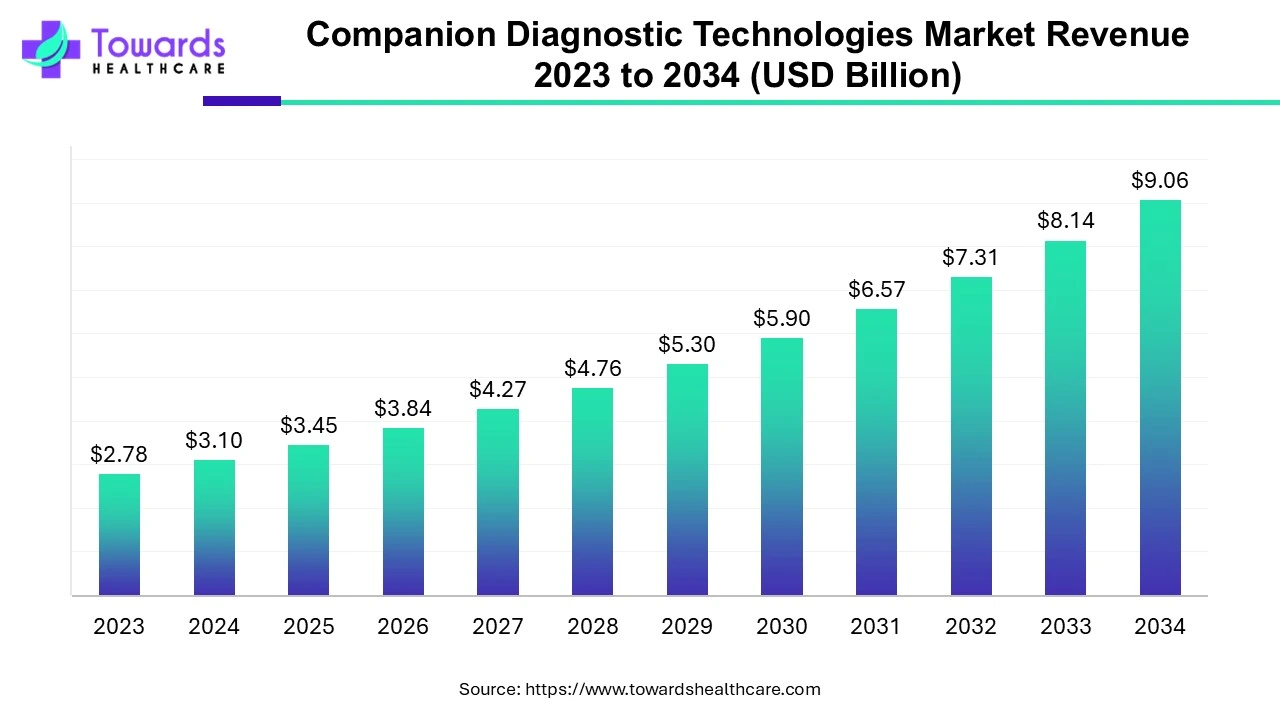
Companion Diagnostic Technologies Market: Driving Personalized Treatment
Companion diagnostics (CDx) are tests that help determine the most appropriate and effective use of a specific drug or biological product. These tests identify which patients are most likely to benefit from a particular treatment. CDx can involve various tools, including in vitro diagnostic (IVD) devices or imaging technologies. These tests are conducted on biological samples, such as tissue biopsies, blood, plasma, or bone marrow, and utilize advanced genomic methods like next-generation sequencing (NGS) or qPCR, along with protein-based technologies like immunohistochemistry.
The market for CDx is being driven by the growing demand for personalized medicine and the rise of targeted therapies. CDx tests allow for more tailored treatments across various diseases, offering insight into potential side effects and the therapeutic effects of medications. As research and development in this field continue to expand, along with the increasing number of clinical trials for cancer and other chronic diseases, the adoption of CDx is expected to grow.
Companion Diagnostic Technologies Market Trends
- In September 2024, Agilent Technologies launched its Biopharma CDx Services Lab in California, aiming to streamline the transition from early assay development and testing to full commercialization of companion diagnostics.
- In August 2024, Becton, Dickinson, and Company announced a strategic partnership with Quest Diagnostics to co-develop, manufacture, and market flow cytometry-based companion diagnostics for cancer and other diseases.
- In April 2023, Foundation Medicine partnered with Bristol Myers Squibb to develop the FoundationOne CDx test as a companion diagnostic for repotrectinib, an experimental tyrosine kinase inhibitor.