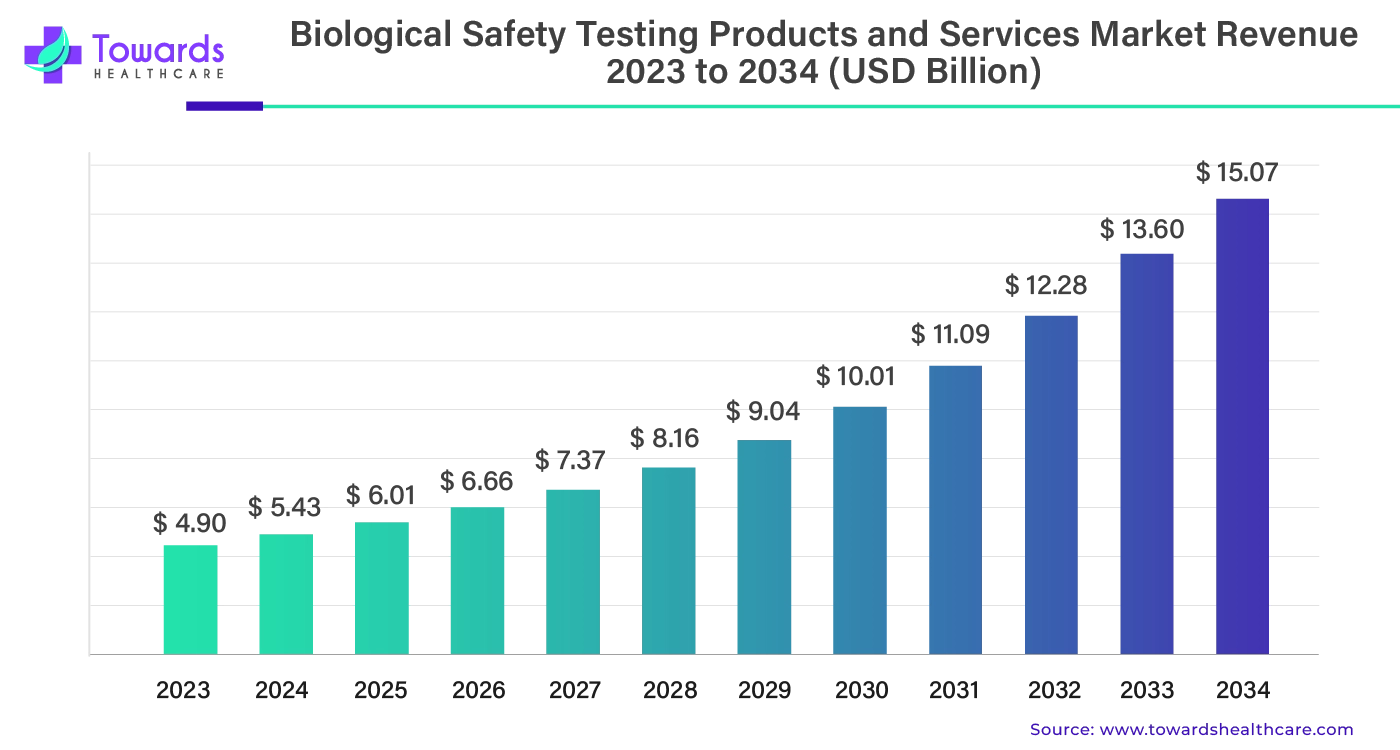
The global biological safety testing products and services market was valued at approximately USD 4.90 billion in 2023 and is projected to grow to USD 15.07 billion by 2034, reflecting a compound annual growth rate (CAGR) of 10.75% from 2024 to 2034. This growth is driven by increasing concerns about product safety in the pharmaceutical and biotech industries.
Download a sample of this report @ https://www.towardshealthcare.com/personalized-scope/5209
Key Market Insights
- North America held the largest market share of 36% in 2023.
- The Asia Pacific region is expected to witness the fastest growth during the forecast period.
- The Reagents & Kits segment was the largest market share by product in 2023.
- Vaccines and Therapeutics led the market by application in 2023.
- The Gene Therapy segment is anticipated to grow at the fastest rate by application.
- By test type, the Endotoxin Tests segment held the dominant market share in 2023.
How AI is Transforming Biological Safety Testing
AI is revolutionizing biological safety testing by enhancing predictive analytics, enabling proactive risk management, and improving health outcomes. The integration of AI with digital technologies in biological safety assessments allows for new insights-driven decision-making, increasing precision and efficiency. This fosters innovation, boosts productivity, and positions businesses for long-term success in a rapidly evolving market.
Top Companies in the Biological Safety Testing Market
- Charles River Laboratories
- Lonza
- BIOMÉRIEUX
- SGS Société Générale de Surveillance SA
- Eurofins Scientific
- Samsung Biologics
- FUJIFILM Wako Pure Chemical Corporation
- Thermo Fisher Scientific Inc.
- Sartorius AG
- BSL Bioservice
- Merck KGaA
Recent Innovations by Key Market Players
- Merck KGaA:
- Headquarters: New Jersey, U.S., North America
- Innovation: In April 2024, Merck introduced the Aptegra™ CHO genetic stability assay using whole genome sequencing and analytics, a comprehensive genetic stability test to expedite biosafety testing and commercial production.
- FUJIFILM Wako Pure Chemical Corporation:
- Headquarters: Tokyo, Japan, Asia Pacific
- Innovation: In June 2024, FUJIFILM launched the LumiMAT™ Pyrogen Detection Kit and the PYROSTAR™ Neo+ recombinant protein reagent for in vitro pyrogen and bacterial endotoxin testing.
Market Drivers and Challenges
- Drivers:
- Increased investment in pharmaceutical industries due to rising health concerns, particularly chronic conditions.
- Efforts by private organizations and governments to boost medicine and therapeutic production.
- Challenges:
- Strict government regulations that require significant investment in advanced testing infrastructure and equipment to reduce bio-risks.
Technological Advancements Shaping the Future
Advancements in technology, such as improved systems and equipment, are expected to significantly enhance the biological safety testing products and services market. These improvements will lead to greater accuracy, reduced testing time, minimized human errors, and increased safety, thereby alleviating regulatory burdens and speeding up the development of new medicines and therapeutics.
Market Segments
- By Product:
- Reagents & Kits
- Instruments
- Services
- By Application:
- Vaccines & Therapeutics (including Monoclonal Antibodies, Recombinant Proteins, Gene Therapy, Blood & Blood-based Products, Tissue & Tissue-based Products, Stem Cells)
- By Test Type:
- Endotoxin Tests
- Bioburden Tests
- Sterility Tests
- Cell Line Authentication & Characterization Tests
- Adventitious Agent Detection Tests
- Residual Host Contamination Detection Tests
- By Region:
- North America (U.S., Canada)
- Asia Pacific (China, Japan, India, South Korea, Thailand)
- Europe (Germany, UK, France, Italy, Spain, Sweden, Denmark, Norway)
- Latin America (Brazil, Mexico, Argentina)
- Middle East and Africa (MEA) (South Africa, UAE, Saudi Arabia, Kuwait)
Regional Growth Highlights
- North America: Dominated the market in 2023 with a 36% share, driven by a strong pharmaceutical industry, supportive government policies, and advanced healthcare systems.
- Asia Pacific: Expected to grow at the fastest rate, with countries like China and India playing significant roles due to their large populations, growing demand for safe therapeutics, and cost-effective outsourcing capabilities.
Discover our detailed Table of Contents (TOC) for the Industry, providing a thorough examination of market segments, material, emerging technologies and key trends. Our TOC offers a structured analysis of market dynamics, emerging innovations, and regional dynamics to guide your strategic decisions in this rapidly evolving healthcare field – https://www.towardshealthcare.com/table-of-content/biological-safety-testing-products-and-services-market-sizing
Read Also :https://www.healthcarewebwire.com/microrna-market-size/
To own our research study instantly, Click here @ https://www.towardshealthcare.com/price/5209
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Explore the comprehensive statistics and insights on healthcare industry data and its associated segmentation: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare