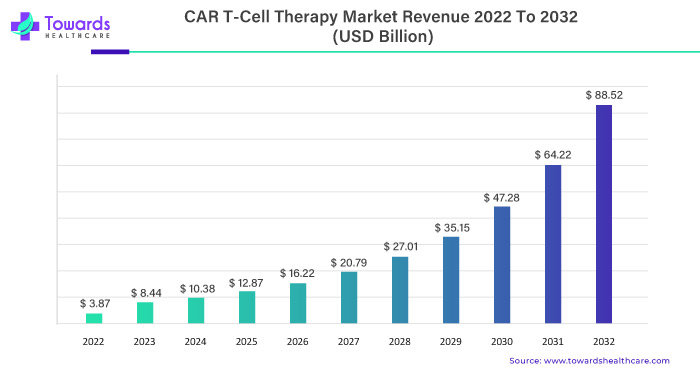
The global CAR T-Cell therapy market is estimated to grow from USD 3.87 billion in 2022 at 29.8% CAGR (2023-2032) to reach an estimated USD 88.52 billion by 2032, as a result of extensive clinical trials and the rising prevalence of cancer.
Download a sample of this report @ https://www.towardshealthcare.com/download-statistics/5028
The United States and China reported most of the trials on Clinicaltrials.gov while Europe lags behind in terms of CAR T-Cell clinical trials.
CAR T-cell therapies have significant transformative potential in the field of cancer treatment as they harness the power of the immune system to fight cancer. These therapies involve genetically engineering T cells to express a chimeric antigen receptor that specifically targets a tumor antigen, providing a highly personalized approach to cancer treatment.
CAR-T therapy is a type of cell therapy in which immune T cells are modified genetically to attack the cancer cells by means of injecting chimeric antigen receptors. It is also referred to as a “miracle anticancer drug” due to its high response rate. It does, however, have a complicated manufacturing process that includes collecting the patient’s T cells at a medical facility and then culturing them in a good manufacturing practice (GMP) facility. The advantages of CAR T-cell therapies over standard medicines include the use of patients’ immune systems to destroy cancer cells, early recovery, and shorter treatment times. Furthermore, CAR T-cell therapy has a long survival time in the body because it has the ability to recognize and target cancer cells even if cancer recurs. CAR T-cell therapy is primarily used to treat lymphoma, acute lymphocytic leukemia, and multiple myeloma. Due to their numerous advantages over traditional drugs, CAR T-cell therapy products have a high adoption rate, resulting in market growth.
Accelerating Innovation in CAR T-Cell Therapy
The rapid pace of innovation in the field of CAR T-cell therapy, and recent product approvals are positive signs for the future of cancer treatment. Extensive R&D has played a significant role in overcoming the limitations of CAR T-cell therapy and improving its efficacy. Strategic collaborations among market players are also fostering the growth of the CAR T-Cell therapy market. The development of novel CAR constructs, new methods for T-cell expansion, and the discovery of new tumor-specific antigens have all contributed to the advancement of CAR T-cell therapy. In addition, improvements in manufacturing processes, such as the use of automated closed systems and cryopreservation, have increased the consistency and quality of CAR T-cell products.
Another critical factor in the development of CAR T-cell therapy is the increase in product approvals. As more products gain approval, the market for CAR T-cell therapy is growing rapidly, creating a competitive landscape that is driving innovation and further research. The FDA has already approved six CAR T-cell therapies for the treatment of certain types of blood cancers, and numerous other CAR T-cell therapies are in clinical trials.
Furthermore, researchers are exploring the use of CAR T-cell therapy in solid tumors. Unlike blood cancers, solid tumors have a more complex microenvironment that can limit the effectiveness of CAR T-cell therapy. Overcoming these challenges will require further research, but the potential benefits of CAR T-cell therapy in solid tumors are substantial.
- In January 2023, CARsgen Therapeutics and Huadong Medicine collaborated to market zevorcabtagene autoleucel (zevor-cel), CT053, in mainland China. In accordance with the agreement, CARsgen received a $29.7 million (RMB 200 million) initial investment and was qualified for commercial and regulatory milestones worth up to $152.4 million (RMB 1,025 million)
- In December 2022, CARsgen Therapeutics Holdings Limited unveiled the findings of its phase I/II LUMMICAR STUDY 1 clinical trial of zevorcabtagene autoleucel. The study involved 14 participants, and results from the first phase revealed a well-tolerated safety profile, as well as substantial and long-lasting responses, with an objective response rate (ORR) of 100% and a complete response/stringent complete response (CR/sCR) rate of 78.6%
- In December 2022, Arcellx, Inc. entered into a global strategic partnership to jointly develop and commercialize its lead late-stage product, CART-ddBCMA, in collaboration with another entity. This partnership aimed to advance the treatment of patients who are suffering from relapsed or refractory multiple myeloma.
- In August 2022, Poseida Therapeutics, Inc. and Roche collaborated to develop CAR-T therapies for hematologic malignancies.
The COVID-19 Effect
Prior to COVID-19, the industry for CAR-T cell treatment was expanding quickly and receiving considerable investment from pharmaceutical firms. For juvenile acute lymphoblastic leukemia, the FDA approved the first CAR-T cell therapy in 2017, and since then, numerous other CAR-T cell therapies have been approved for various disease types. Novartis and Gilead Sciences had the majority of the market share for CAR-T cell therapy, dominating the market. Several businesses, such as Kite Pharma, Juno Therapeutics, and Bluebird Bio, were working on CAR-T cell therapy, though.
The market for CAR-T cell treatment has been significantly impacted by the COVID-19 epidemic. Clinical trial delays, supply chain interruptions, and a decline in the number of patients seeking treatment have all been brought on by the epidemic. More patients are turning to telemedicine and other forms of remote care as a result of the epidemic, which has also changed how healthcare is delivered. In response to the epidemic, some businesses have increased their manufacturing capacity to accommodate the rising demand for CAR-T cell therapy. Others have concentrated on creating novel therapies that may be delivered remotely, minimizing the number of visits that patients must make to clinics and hospitals.
Segments Covered in the Report
By Drug Type
- Axicabtagene Ciloleucel
- Tisagenlecleucel
- Brexucabtagene Autoleucel
- Others
By Indication
- Lymphoma
- Acute Lymphocytic Leukemia
- Chronic Lymphocytic Leukemia (CLL)
- Multiple Myeloma (MM)
- Others
By End User
- Hospitals
- Cancer Treatment Centers
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
Examine closely: https://www.healthcarewebwire.com/503a-us-compounding-pharmacies-market/
Discover our detailed Table of Contents (TOC) for the Industry, providing a thorough examination of market segments, material, emerging technologies and key trends. Our TOC offers a structured analysis of market dynamics, emerging innovations, and regional dynamics to guide your strategic decisions in this rapidly evolving healthcare field – https://www.towardshealthcare.com/table-of-content/car-t-cell-therapies-are-poised-for-the-next-wave-of-innovation
To own our research study instantly, Click here @ https://www.towardshealthcare.com/price/5028
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Explore the comprehensive statistics and insights on healthcare industry data and its associated segmentation: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare