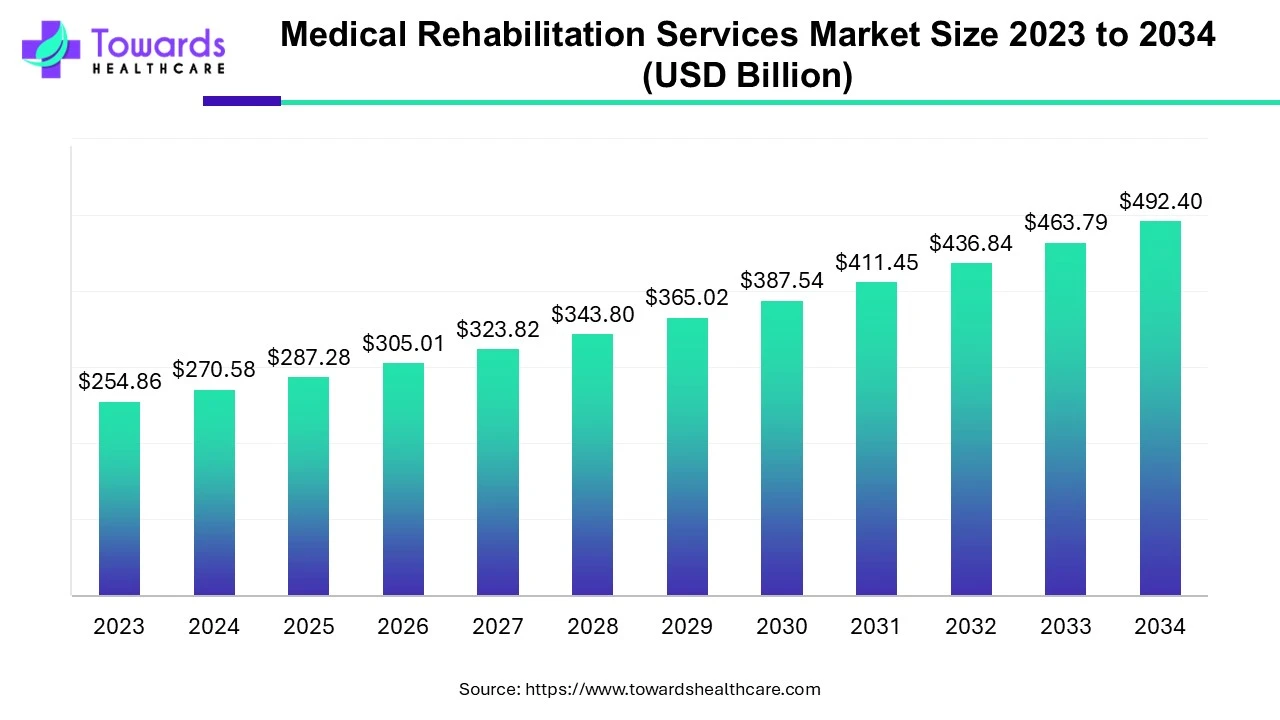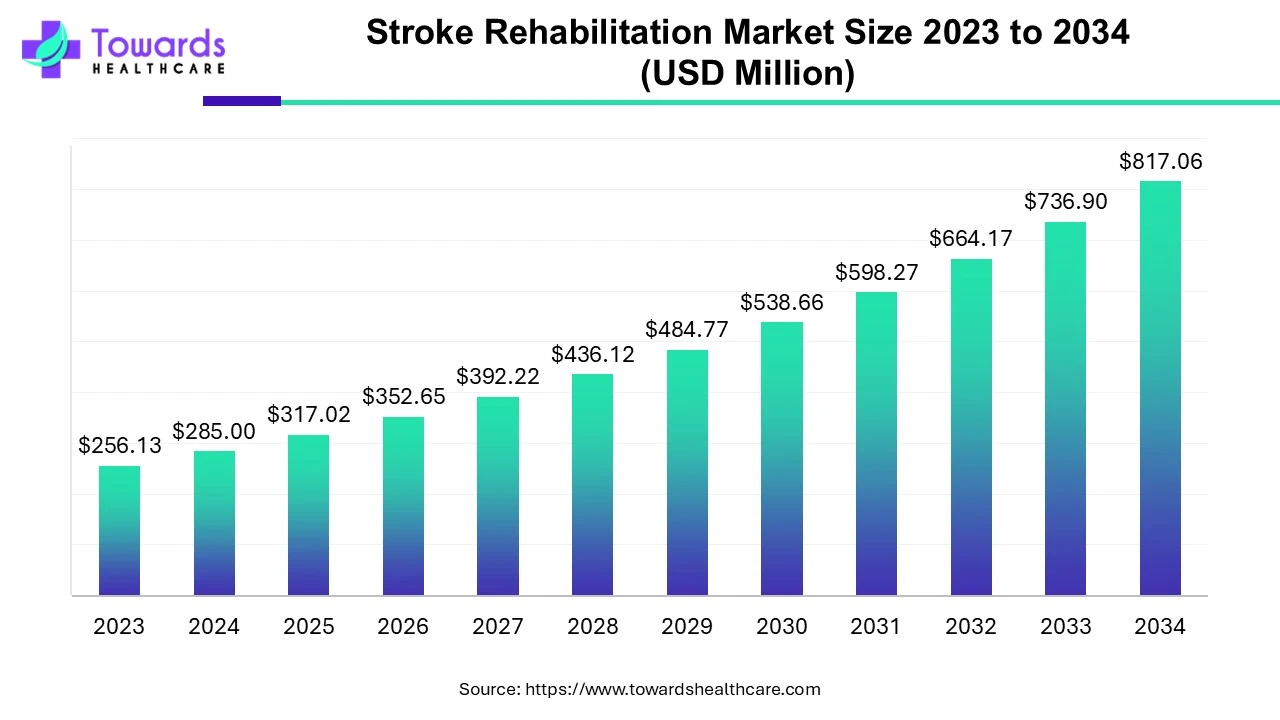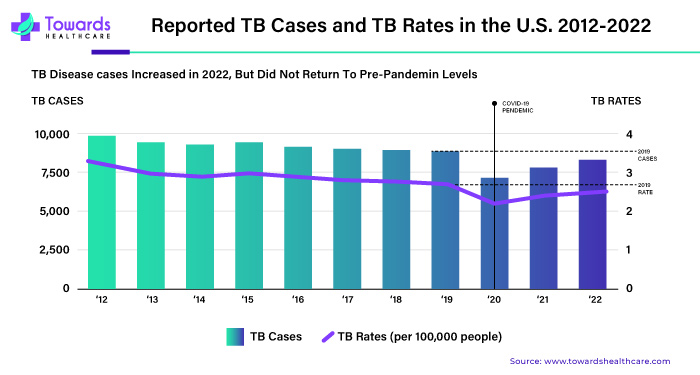
Thermostable vaccines represent a remarkable advancement in medical science, offering enhanced accessibility and efficacy in combating infectious diseases. However, before these groundbreaking vaccines can be deployed for widespread use, they must undergo rigorous regulatory approval processes to ensure their safety, effectiveness, and stability. In this comprehensive guide, we delve into the intricacies of regulatory approvals for thermostable vaccines, emphasizing their critical importance in safeguarding public health.
For any queries, we are available for you @ https://www.towardshealthcare.com/personalized-scope/5117
Understanding Regulatory Standards
Regulatory agencies worldwide impose stringent standards that thermostable vaccines must meet to secure approval for distribution. These standards encompass a multifaceted evaluation, including comprehensive clinical trials to assess efficacy and safety profiles. Additionally, the stability of these vaccines under varying environmental conditions, particularly temperature fluctuations, is meticulously scrutinized.
Clinical Trials and Safety Assessments
Clinical trials serve as the cornerstone of regulatory approval processes, providing pivotal insights into the performance and safety profiles of thermostable vaccines. These trials involve meticulous testing on human subjects to ascertain the vaccine’s ability to induce robust immune responses while minimizing adverse reactions. Rigorous monitoring and data analysis throughout the trial phases ensure the integrity and reliability of the findings.
Stability Testing and Assurance
Ensuring the stability of thermostable vaccines is paramount to their viability and effectiveness, especially in regions with limited access to refrigeration infrastructure. Regulatory authorities mandate extensive stability testing to assess the vaccine’s resilience to temperature variations over time. This involves subjecting vaccine samples to accelerated aging studies and real-time storage conditions to simulate real-world scenarios and validate their efficacy.
The Approval Process: A Complex Journey
Navigating the regulatory approval pathway for thermostable vaccines is a complex and time-intensive endeavor fraught with challenges and intricacies. Vaccine developers must compile a robust dossier of scientific data, including preclinical studies, clinical trial results, and manufacturing protocols, to support their application for approval.
Submission and Review Process
Once the requisite data is compiled, vaccine developers submit their regulatory applications to the appropriate authorities, initiating a meticulous review process. Regulatory agencies meticulously scrutinize every aspect of the submitted data, evaluating its scientific validity, compliance with regulatory standards, and potential risks and benefits to public health.
Timeline and Challenges
The timeline for regulatory approval of thermostable vaccines can vary significantly depending on numerous factors, including the complexity of the vaccine, the completeness of the data package, and the regulatory agency’s workload. While some vaccines may receive expedited review and approval, others may face prolonged deliberations, delaying their availability for public use.
Implications of Regulatory Delays
The protracted nature of the regulatory approval process for thermostable vaccines can have profound implications for public health outcomes. Delays in approval translate to delays in vaccine availability, exacerbating the burden of preventable diseases and impeding efforts to curtail outbreaks. Moreover, the financial burden associated with prolonged regulatory reviews poses significant challenges for vaccine developers, particularly smaller organizations with limited resources.
Collaboration and Streamlining Efforts
Addressing the challenges inherent in the regulatory approval of thermostable vaccines necessitates collaborative efforts among stakeholders, including vaccine developers, regulatory agencies, public health organizations, and policymakers. Streamlining regulatory processes, fostering innovation in vaccine development, and leveraging technological advancements can expedite approvals and facilitate timely access to life-saving vaccines.
Public Health Imperative
Despite the formidable challenges posed by the regulatory approval process, ensuring the safety, efficacy, and stability of thermostable vaccines remains a paramount public health imperative. Regulatory agencies play a pivotal role in safeguarding public health by upholding stringent standards and meticulous oversight, thereby instilling confidence in the integrity and reliability of approved vaccines.
In regulatory approvals for thermostable vaccines represent a pivotal milestone in the journey towards enhancing global health outcomes. By adhering to rigorous standards, conducting robust clinical trials, and fostering collaborative partnerships, stakeholders can accelerate the approval process and expedite the deployment of life-saving vaccines to populations in need. Upholding the highest standards of safety, efficacy, and stability ensures that thermostable vaccines fulfill their promise of safeguarding public health and combating infectious diseases effectively.
To own our premium research study instantly, click here @ https://www.towardshealthcare.com/price/5117
Unlock Infinite Advantages: Subscribe to Annual Membership
Read More Information Related to Thermostable Vaccine Sector: