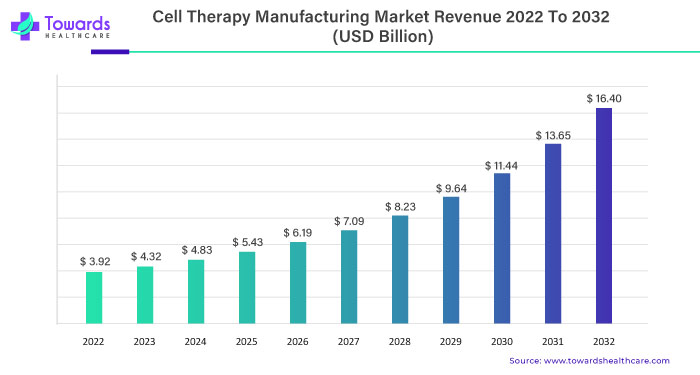
In the ever-evolving landscape of healthcare, the global cell therapy manufacturing market is poised for substantial growth. Projections indicate a robust 16% Compound Annual Growth Rate (CAGR) from 2023 to 2032, propelling the market from its 2022 valuation of USD 3.92 billion to an estimated USD 16.40 billion by 2032.
Nearly all cancer diagnoses, accounting for 90% of cases, require cell therapies to effectively treat solid tumors.
Cell therapy manufacturing market refers to the process of producing therapeutic cells that are used for the treatment of various diseases and medical conditions. It involves the isolation, expansion, modification, and quality control of cells in order to generate a sufficient number of functional cells for therapeutic use. Cell therapies have gained significant attention in recent years due to their potential for treating a wide range of diseases, including cancer, cardiovascular disorders, neurodegenerative diseases, and autoimmune disorders.
The manufacturing process for cell therapies is complex and requires specialized infrastructure, equipment, and expertise. It typically involves the use of advanced techniques such as cell culture, genetic engineering, and quality control testing. The cells used in therapy can be derived from different sources, including autologous (patient’s own cells) or allogeneic (donor-derived) cells.
The driving factors for the cell therapy manufacturing market include the increasing demand for personalized medicine, advancements in cell therapy research and development, and the growing prevalence of chronic diseases. Cell therapies offer the potential for targeted and individualized treatments, which can lead to improved patient outcomes. Additionally, the rising investments in regenerative medicine and the favorable regulatory environment for cell therapies further drive market growth.
Collaborative Growth: Expanding Horizons in the Cell Therapy Manufacturing Market
The cell therapy manufacturing market is witnessing a significant increase in collaborations and expansion activities by market players, which is driving its growth. Collaborations between academic institutions, research organizations, and industry players are becoming increasingly common in the field of cell therapy manufacturing. These partnerships aim to combine resources, expertise, and technologies to accelerate the development and commercialization of cell therapies.
Through collaborations, companies can access a broader range of knowledge and capabilities, enabling them to overcome technical challenges and optimize their manufacturing processes. They can also leverage the expertise of academic and research institutions to enhance their understanding of cell biology, process optimization, and quality control. By pooling resources and sharing risks, collaborations help to expedite the translation of cell therapies from the laboratory to the clinic. For instance.
- In May 2023, Thermo Fisher Scientific and the University of California, San Francisco (UCSF) announced a partnership to establish a new cell therapy cGMP manufacturing site. This facility will be located adjacent to UCSF Medical Center’s Mission Bay Campus. The collaboration aims to combine Thermo Fisher Scientific’s expertise in cell therapy manufacturing and UCSF’s research and clinical capabilities to accelerate the development and production of innovative cell-based therapies.
- In March 2023, Cell One Partners and the Center for Breakthrough Medicines (CBM) entered into a strategic collaboration aimed at accelerating the development and commercialization of cell and gene therapies. This collaboration brings together the expertise and resources of both organizations to drive innovation and advance the field of regenerative medicine.
- In September 2022, the Cell Therapy Manufacturing Center (CTMC) and Ori Bio collaborated to expedite the process development, commercialization, and clinical integration of cell therapies. This partnership aimed to enhance the advancement of cell-based treatments and bring them to patients more efficiently.
- In March 2022, Novartis entered into an initial agreement with Carisma Therapeutics, a biopharmaceutical company specializing in engineered macrophage-based therapeutics. The agreement involves the manufacturing of the HER 2 targeted CAR-M cell therapy, which is currently being tested in initial trials for the treatment of solid tumors. This collaboration aims to leverage Carisma Therapeutics’ expertise in engineered macrophages and Novartis’ capabilities in cell therapy manufacturing market to advance the development of innovative treatments for solid tumors.
In addition to collaborations, market players are expanding their manufacturing capabilities through facility expansions and acquisitions. The growing demand for cell therapies and the need for increased production capacity is driving companies to invest in larger and more advanced manufacturing facilities. These expansions not only enable companies to meet the rising demand for cell therapies but also provide them with the infrastructure and resources to support further research and development.
For instance, in April 2023, Bristol Myers Squibb revealed plans to expand its global cell therapy manufacturing market network. This expansion includes the establishment of a U.S.-based manufacturing facility in Libertyville, Illinois, to facilitate in-house viral vector production. The company executed an agreement with Novartis to support this initiative, which aims to enhance Bristol Myers Squibb’s capabilities in cell therapy manufacturing market.
Expanding into new geographies is another strategy adopted by market players to capitalize on emerging markets and gain a competitive edge. By establishing manufacturing facilities or partnerships in different regions, companies can cater to local patient populations, leverage regional expertise, and comply with specific regulatory requirements. This expansion allows companies to access new markets, increase their market share, and diversify their revenue streams.
The rising collaborations and expansions in the cell therapy manufacturing market are driven by several factors. Firstly, the increasing demand for cell therapies and the growing pipeline of cell therapy candidates are creating opportunities for market players to scale up their manufacturing operations. The potential of cell therapies to revolutionize healthcare and provide innovative treatment options is attracting significant investments and industry interest.
Trials Fueling Transformation: Market Growth Driven by Clinical Trials and Product Launches in Cell Therapy Manufacturing Market
Clinical trials play a crucial role in driving market growth in the field of cell therapy manufacturing market. Clinical trials are conducted to assess the safety and efficacy of cell therapies in humans. These trials provide valuable data on the therapeutic potential, dosage, administration, and potential side effects of cell therapies. Positive results from clinical trials contribute to market growth by building confidence among healthcare professionals, regulatory authorities, and patients. Clinical trials are a prerequisite for obtaining regulatory approvals for cell therapies.
Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), require robust clinical trial data to evaluate the safety and efficacy of cell therapies before granting market authorization. Successful completion of clinical trials and obtaining regulatory approvals pave the way for commercialization and market entry. The rising number of clinical trials significantly augments the growth of this market. For instance, in July 2021, the U.S. National Library of Medicine published a study document focusing on Apheresis for CAR T-Cell Manufacturing. The study delves into the use of apheresis, a medical procedure involving the removal and separation of specific components from blood, for the manufacturing of CAR T-cells.
In addition, clinical trials enable researchers and developers to explore new indications and expand the application of cell therapies. Through well-designed clinical trials, the efficacy of cell therapies can be evaluated in various disease areas, leading to the expansion of treatment options and addressing unmet medical needs. The identification of new indications and successful clinical trial outcomes contribute to market growth. Positive outcomes from clinical trials provide a competitive advantage to cell therapy manufacturers.
Demonstrating the safety and efficacy of their therapies through well-designed clinical trials helps companies differentiate themselves from competitors and gain market share. Clinical trial data strengthens the credibility of cell therapies and enhances their market appeal among healthcare providers, payers, and patients. Increasing product launches and product approvals extensively bolster the growth of the cell therapy manufacturing market. For instance, in March 2022, Merck KGaA introduced EX-CELL CD Insect Cell Medium a chemically defined medium specially formulated to get the best performances for insect cell lines. It provides the necessary nutrients, growth factors, and buffering agents required for optimal cell growth and productivity.
Precision in Production: The Engine of Cell Therapy Manufacturing
Revolutionizing Treatment Modalities
Cell therapy manufacturing stands at the forefront of transforming healthcare paradigms. This industry’s growth is underpinned by advancements in manufacturing processes that enable the large-scale production of cell-based therapies. As precision in production increases, so does the potential to address a myriad of medical conditions with innovative cellular therapies.
Meeting the Rising Demand
The increasing prevalence of cell-based therapies as a viable treatment option fuels the demand for efficient manufacturing processes. Streamlining production not only meets the current demand but positions the industry to accommodate future therapeutic breakthroughs, ensuring a scalable and responsive manufacturing infrastructure.
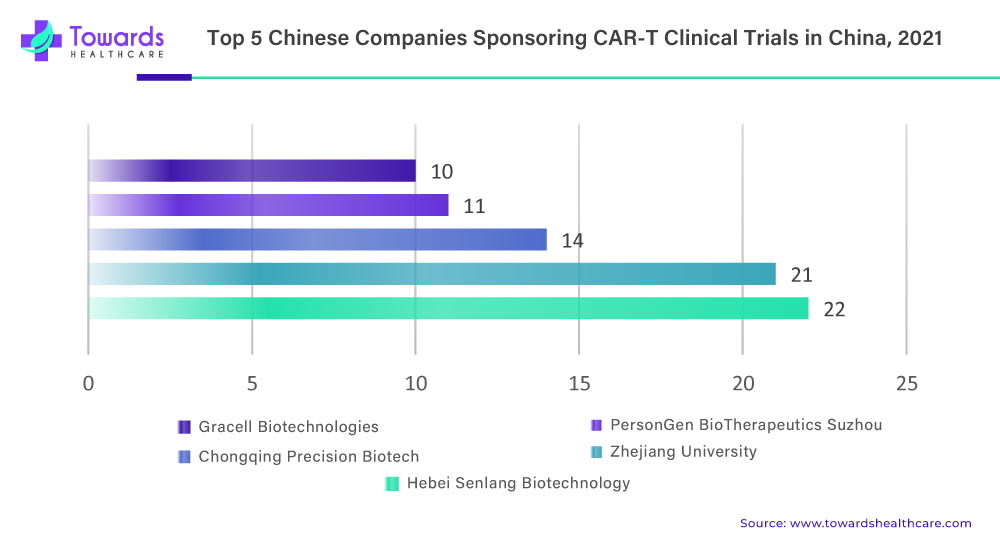
In Beijing, CAR-T research involves various entities such as universities, clinical researchers, and small start-up companies. However, many of these entities lack the infrastructure to build their own manufacturing capacity. To address this issue, the Beijing government has set up a centralized production line in the metropolitan science and technology park. This facility offers manufacturing space that can be rented by researchers and start-ups, aiming to lower their research and development costs.
This initiative provides a solution for entities involved in CAR-T research to access manufacturing capabilities and promote the advancement of their therapies.
Clinical trials are essential drivers of the cell therapy manufacturing market. They provide evidence of safety and efficacy, support regulatory approvals, facilitate treatment optimization and innovation, differentiate products in the market, attract investors, and enable the exploration of new indications. Continued investment in well-designed clinical trials is crucial for the growth and advancement of the cell therapy manufacturing market.
Cost-Effective Innovations: Addressing Development Costs in Cell Therapy Manufacturing Market
Developing cell therapies requires extensive research and development efforts, including preclinical studies and early-phase clinical trials. These activities involve significant investment in laboratory equipment, personnel, materials, and specialized facilities. Establishing the necessary manufacturing infrastructure for cell therapies can be costly. This includes cleanroom facilities, specialized equipment, and quality control systems to ensure the safety and efficacy of the manufactured products. The costs of maintaining and operating these facilities add to the overall development expenses.
Conducting clinical trials is a resource-intensive process. Patient recruitment, treatment administration, monitoring, data collection, analysis, and reporting all contribute to the high costs. In addition, complying with regulatory requirements and ensuring patient safety through rigorous monitoring and adverse event reporting further adds to the expenses. Scaling up cell therapy production from small-scale clinical trials to large-scale commercial manufacturing involves substantial costs. The costs associated with process optimization, facility expansion, and quality control measures increase as the production volume increases.
Meeting regulatory standards and ensuring product quality and safety throughout the manufacturing process require robust quality control measures. These measures can be expensive to implement and maintain, including testing, validation, documentation, and compliance with good manufacturing practices (GMP) regulations. Protecting intellectual property rights through patents and licensing agreements is crucial in the cell therapy field. Securing and maintaining intellectual property rights involves legal fees and ongoing costs, which can be significant.
The high development costs pose challenges for both industry players and academic institutions, especially for small and emerging companies with limited financial resources. These costs may deter some companies from entering the market or hinder the progress of research and development initiatives.
To address this restraint, it is important to explore strategies to optimize the development process, improve efficiency, and reduce costs. Collaboration between industry, academia, and regulatory bodies can help in streamlining regulatory requirements and fostering an environment conducive to cost-effective development. Additionally, investments in research and development funding, government incentives, and public-private partnerships can support the advancement of cell therapy manufacturing market and mitigate the financial burden on stakeholders.
Advancements in Technology and Manufacturing Processes Unleash Opportunities in the Cell Therapy Market
Advancements in technology and manufacturing processes have played a significant role in unlocking opportunities in the cell therapy market. These advancements have revolutionized the field of cell therapy, enabling the development and manufacturing of innovative and complex therapies. Technological advancements have led to the development of automated and closed-system manufacturing platforms, allowing for the production of cell therapies at a larger scale. This scalability improves efficiency, reduces costs, and increases the availability of cell therapies for a broader patient population.
Advanced bioreactors, monitoring systems, and analytics tools have enabled better process control and optimization in cell therapy manufacturing market. This optimization improves product quality, consistency, and reproducibility, reducing the risk of batch failures and increasing the overall success rate of cell therapies. innovations in gene editing technologies, such as CRISPR-Cas9, have opened up new possibilities for modifying and engineering cells used in therapies. This technology allows for precise and targeted modifications of cell genomes, enhancing therapeutic efficacy and expanding the scope of cell therapies.
Cryopreservation technologies have improved the long-term storage and preservation of cells, enabling their use in off-the-shelf therapies. Cryopreserved cell products offer convenience and flexibility in clinical settings and facilitate wider adoption of cell therapies. Advances in technology have facilitated the development of combination therapies that combine different cell types, gene therapies, or small molecules. These synergistic approaches have the potential to enhance therapeutic outcomes and address complex diseases more effectively.
Technological advancements, such as high-throughput screening and next-generation sequencing, have enabled the characterization of patient-specific cells and identification of biomarkers. This personalized approach allows for the development of tailored cell therapies and more precise treatment strategies.
Thus, advancements in technology and manufacturing processes have revolutionized the cell therapy market, offering numerous opportunities for innovation and growth. By leveraging these advancements, companies can enhance the scalability, cost-efficiency, and efficacy of cell therapies, driving the field forward and improving patient outcomes.
North America Takes the Lead: Dominance in the Cell Therapy Manufacturing Market
North America, particularly the United States, holds a significant share in the cell therapy manufacturing market. The region benefits from advanced healthcare infrastructure, a well-established regulatory framework, and a strong presence of key market players. The United States, in particular, has been at the forefront of cell therapy research and development, attracting substantial investments and fostering collaborations between industry, academia, and research institutions.
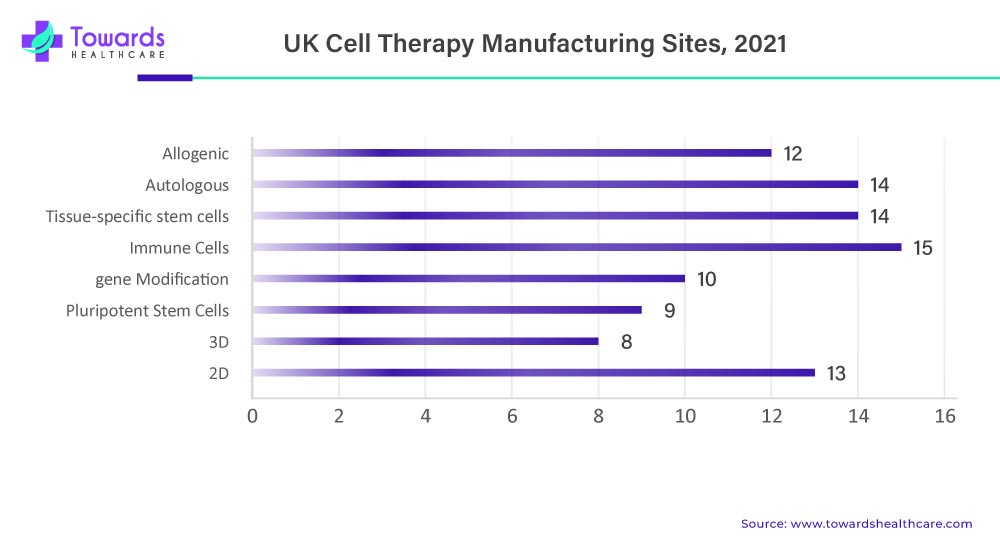
In addition, Europe is another prominent region in the cell therapy manufacturing market. Countries such as Germany, the United Kingdom, and France have a robust healthcare system and a supportive regulatory environment for cell therapies. The European Medicines Agency (EMA) provides guidelines and regulations for the development and commercialization of cell therapies, ensuring safety and efficacy. The region also witnesses collaborations and partnerships among academic institutions, hospitals, and industry players to advance cell therapy manufacturing market.
Cell Therapy Manufacturing Market Facilities in the U.K.
- Instil Bio UK (formerly Immetacyte Ltd)
- John Goldman Centre for Cellular Therapy, Imperial College London
- Moorfields Eye Hospital, Cells for Sight Stem Cell Therapy Research Unit
- National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St
- Thomas’ NHS Foundation Trust and King’s College London (GSTT BRC)
- Newcastle University, Newcastle Cellular Therapies Facility
- NHSBT Barnsley
- NHSBT Birmingham
- NHSBT Speke
- Scottish National Blood Transfusion Service (SNBTS)
- University of Birmingham, Advanced Therapies Facility
Furthermore, the Asia-Pacific region is experiencing significant growth in the cell therapy manufacturing market. Countries like China, Japan, and South Korea are actively investing in research and development and building advanced manufacturing facilities. The region benefits from a large patient population, increasing healthcare expenditure, and supportive government initiatives to promote the development and adoption of cell therapies. Asia-Pacific is also emerging as a hub for contract manufacturing organizations (CMOs) offering cell therapy manufacturing market services.
The Trajectory of Growth: A 16% CAGR Journey
Charting the Course to 2032
With a projected 16% CAGR, the cell therapy manufacturing market is on an upward trajectory. Investors and stakeholders keen on navigating the landscape of healthcare manufacturing should be attuned to emerging trends, technological advancements, and strategic collaborations driving this remarkable journey to reach an estimated USD 16.40 billion by 2032.
Global Impact and Market Expansion
The global reach of cell therapy manufacturing market extends its influence across borders. As the market grows, it not only meets the burgeoning demand for advanced therapies but also establishes itself as a pivotal player in the global healthcare ecosystem. Opportunities for market expansion and collaborative ventures abound, setting the stage for a transformative era in medical manufacturing.
Future-Ready: Investing in Innovation and Infrastructure
Capitalizing on Technological Advancements
The future of the cell therapy manufacturing market hinges on innovation. Technological advancements, automation, and streamlined production processes are catalysts for growth. Investors eyeing the burgeoning market should prioritize opportunities that align with cutting-edge technologies shaping the future of cell therapy manufacturing market.
Infrastructure as the Backbone
A resilient and adaptable infrastructure is key to sustaining the growth momentum. Investment in facilities, equipment, and skilled workforce ensures that the cell therapy manufacturing market remains robust and ready to meet the evolving demands of a dynamic healthcare landscape.
Key Market Players
- Merck KGaA
- Thermo Fisher Scientific
- Catalent, Inc
- Bio-Techne
- Cytiva
- Lonza
- The Discovery Labs
- Novartis AG
- Bristol-Myers Squibb Company
- Gilead Sciences, Inc.
Segments Covered
By Indication
- HIV
- Autoimmune Disorders
- Immune Deficiencies
- Cancer
- Neurological Disorders
By Route of Administration:
- Topical
- Injectable
- Infusion
- Implantable Bio-Scaffold
By Cell Type:
- Hematopoietic (Blood-Forming) Stem Cells (HSC)
- Skeletal Muscle Stem Cells
- Mesenchymal Stem Cells
- Lymphocytes
- Dendritic Cells
- Pancreatic Islet Cells
- CAR-T Cells
By End User:
- Hospital Settings
- Intensive Outpatient Treatment Centers
- Academic and Research Institutes
- Specialty Clinics
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
Conclusion: Navigating the Horizon of Healthcare Manufacturing
In conclusion, the global cell therapy manufacturing market’s trajectory is one of remarkable growth and transformative impact. With a projected valuation of USD 16.40 billion by 2032, this market not only signifies economic potential but also underscores the pivotal role of advanced manufacturing in shaping the future of healthcare. As we navigate the horizon of healthcare manufacturing, the cell therapy sector stands as a beacon of innovation and progress, poised to revolutionize treatment modalities on a global scale.