Companion Diagnostic Technologies Market Key Developments and Growth Drivers

Table of Contents
ToggleCompanion Diagnostic Technologies Market Size Growth and Key Developments
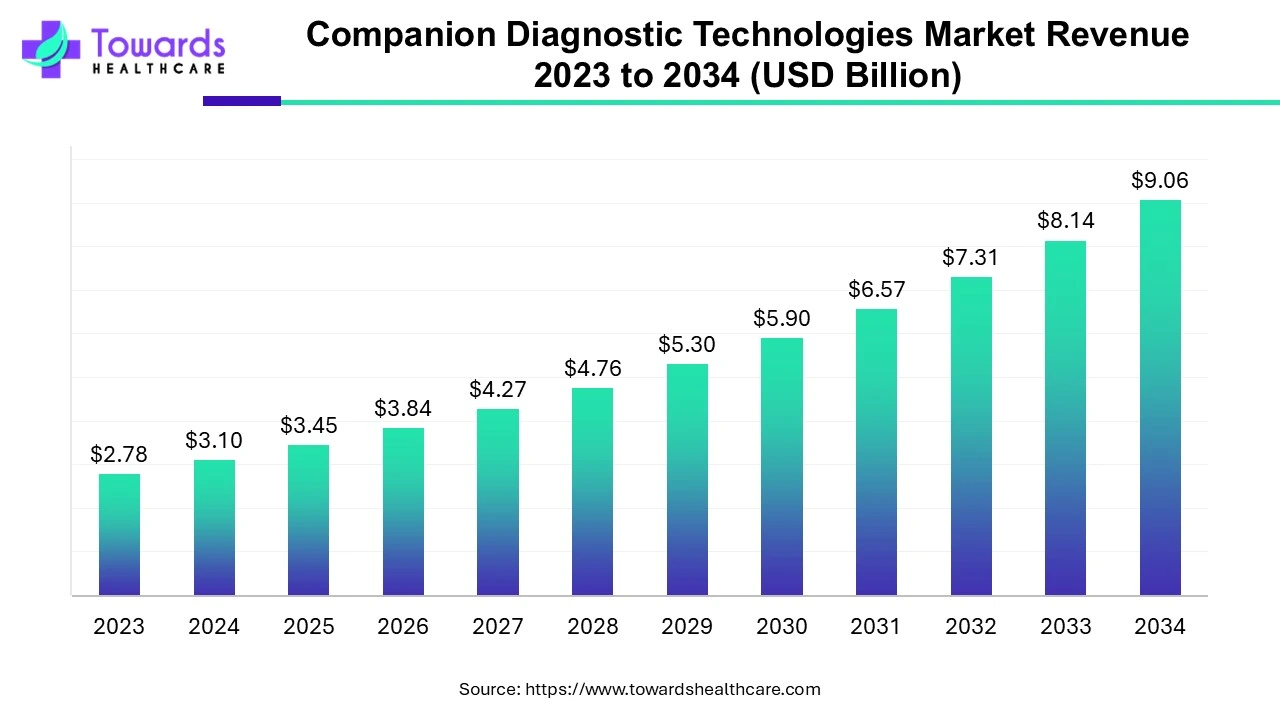
The companion diagnostic technologies market was valued at $2.78 billion in 2023 and is expected to grow to $9.06 billion by 2034, with a strong compound annual growth rate (CAGR) of 11.34% from 2024 to 2034.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5330
Companion Diagnostic Technologies Market: Advancing Personalized Treatment
Companion diagnostics (CDx) are tests designed to help determine the most effective and safest use of a drug or biological product. These tests identify patients who are most likely to benefit from a particular treatment, guiding clinicians toward the best therapeutic options. CDx involves various tools, such as in vitro diagnostic (IVD) devices or imaging techniques, and can be conducted using different biological samples like tissue biopsies, blood, plasma, or bone marrow. The tests employ advanced genomic technologies such as next-generation sequencing and qPCR, as well as protein-based methods like immunohistochemistry.
The market for CDx is driven by the growing demand for personalized medicine and the development of targeted therapies. CDx tests are pivotal in offering more precise, tailored treatments for a range of diseases, providing crucial information about potential side effects and the therapeutic effects of drugs before they are administered. The expanding research efforts and increasing availability of clinical drugs, particularly for cancer and chronic conditions, make CDx an integral part of modern healthcare.
Companion Diagnostic Technologies Market Trends
- In September 2024, Agilent Technologies, Inc. launched its Biopharma CDx Services Lab in California, aimed at supporting the transition from early assay development to full commercialization of companion diagnostics.
- In August 2024, Becton, Dickinson, and Company partnered with Quest Diagnostics to develop and market flow cytometry-based CDx for cancer and other diseases.
- In April 2023, Foundation Medicine joined forces with Bristol Myers Squibb to advance the FoundationOne CDx test, which will serve as a companion diagnostic for repotrectinib, an experimental tyrosine kinase inhibitor.
Top Companies in the Companion Diagnostic Technologies Market
- Agilent Technologies, Inc.
- Abbott Laboratories
- Becton, Dickinson and Company
- BioGenex
- Bristol Myers Squibb
- Danaher Corporation
- Foundation Medicine
- Myriad Genetics, Inc.
- Qiagen NV
- Quest Diagnostics
- Servier Pharmaceuticals
- Siemens Healthineers
- Sysmex Corp
- Tempus Technologies
- Thermo Fisher Scientific
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/companion-diagnostic-technologies-market-sizing
Access exclusive insight now @ https://www.towardshealthcare.com/price/5330
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare