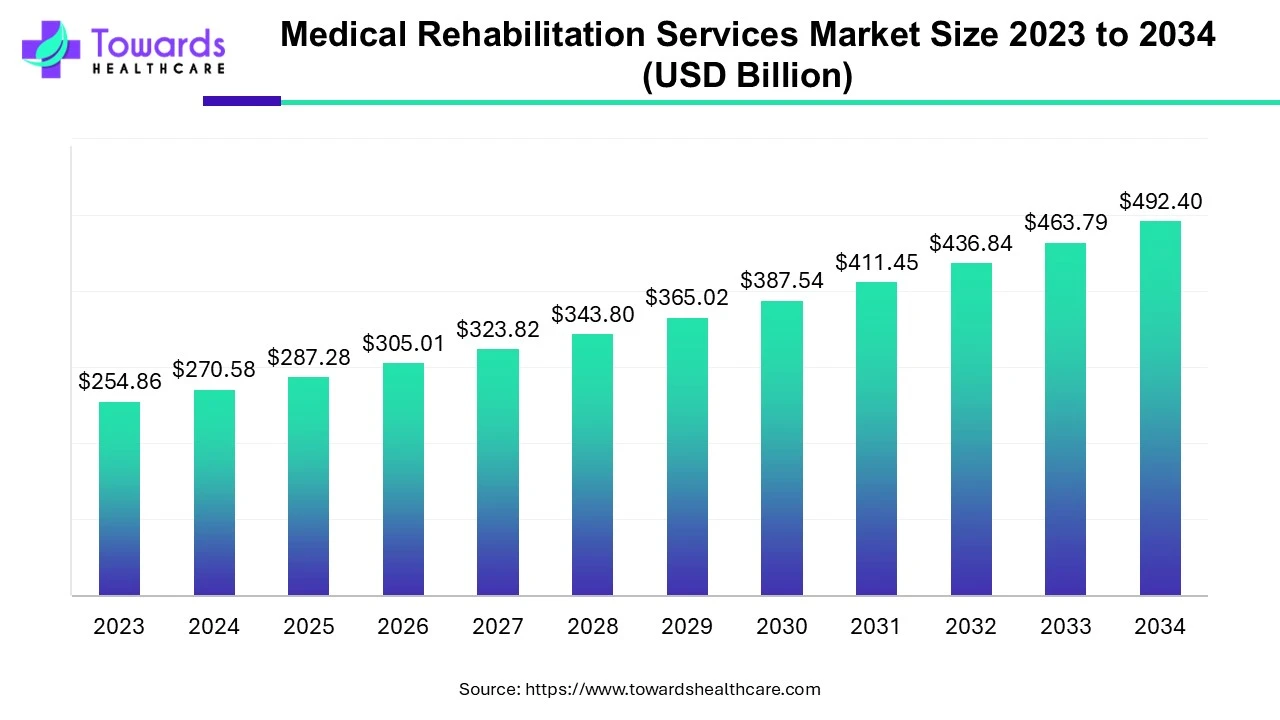
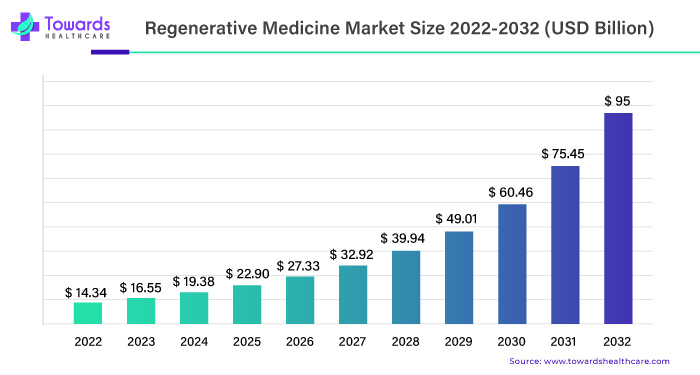
The global regenerative medicine market size is estimated to grow from USD 14.34 billion in 2022 to surpass around USD 95 billion by 2032, expanding at a CAGR of 21.4% between 2023 and 2032, as a result of the rising demand for cell and gene therapies.
Report Highlights:
- North America dominated regenerative medicine, exhibiting around 49.21% market share.
- The wound care segment by application is estimated to be the fastest growing segment with 17.3% CAGR from 2023-2032.
- Nearly 1 in every 3 Americans may benefit from regenerative medicine, as per the current estimates.
- High prevalence of chronic diseases in North America contributes to market dominance.
In September 2023, OrganDonor.gov estimated that there were 15,927 kidney transplants, 6,143 liver transplants and 2,671 heart transplants, driving increased demand for innovative transplants performed by organ for kidney 15,927, for liver 6,143 and for heart 2,671, which creates demand for innovation of regenerative medicine.
Regenerative medicine market arises from addressing organ and tissue loss caused by diseases and injuries, aiming to reduce reliance on transplants. This interdisciplinary field combines engineering and life sciences to facilitate tissue regeneration, with FDA-approved therapies now available commercially. The existing regenerative medicine market approaches and ongoing studies emphasise advancements in graft fabrication, tissue mimics and technologies for graft integration with host vasculature. Strategies to enhance the host’s regenerative capacity through cell injections, immune modulation and newly developed cell sources are also discussed.
The potential of regenerative medicine market extends to repairing or replacing damaged tissues and organs due to age, disease or trauma and correcting congenital disabilities. Promising preclinical and clinical data suggest the ability to treat chronic diseases, acute insults and a diverse range of organ system issues. Overcoming limitations of organ transplantation, such as donor shortages and immune complications, is envisioned through regenerative medicine market strategies.
Regenerative medicine encompasses various strategies, including using materials, de novo generated cells, or their combinations to structurally and functionally replace missing tissues or aid in tissue healing. While adult humans have limited regenerative capacity compared to lower vertebrates, innovative therapies aim to change patients’ physiological environments through materials, living cells or growth factors, enhancing the body’s innate mechanisms.
Increasing research, technological advancements and a rising prevalence of chronic diseases drive the market. Key players include pharmaceutical companies, biotech firms and academic institutions. Market growth is influenced by regulatory approvals, investment in R&D and collaborations within the industry. It holds promise for treating cardiovascular diseases, orthopaedic disorders and neurodegenerative conditions.
Application Insights:
- Wound care segment estimated to be the fastest-growing with 17.3% CAGR from 2023-2032.
- Increasing demand for innovative transplants, including kidney (15,927), liver (6,143), and heart (2,671) transplants, propels regenerative medicine market development.
Emerging Innovation Leads Growth in Regenerative Medicine Market
Over the past two decades, regenerative medicine market has burgeoned into a dynamic industry, witnessing FDA clearance or approval for several therapeutic interventions, which are now commercially accessible. A pivotal aspect of regenerative medicine lies in delivering therapeutic cells that actively contribute to forming and functioning new tissues, constituting a fundamental paradigm in this domain.
These therapeutic cells, used in various approved therapies, come in two primary forms: autologous and allogeneic. They are typically differentiated cells that retain their capacity for proliferation.
For Instance,
- Notable examples include slavery, employing autologous fibroblast for enhancing the appearance of nasolabial fold wrinkles; Celusion, a medical device extracting cells from adipose tissue obtained through liposuction; Epicel, which deploys autologous keratinocytes for severe burn wounds; and the extraction of hematopoietic progenitor and stem cells from cord blood. Using autologous cells often involves creating a new wound site during harvesting, leading to a delay before treatment due to the necessary culture expansion.
- Allogenic cell sources with low antigenicity, like human foreskin fibroblasts used in crafting wound healing grafts (e.g., GINTUIT), enable the production of off-the-shelf tissues on a mass scale and concurrently reduce the risk of adverse immune reaction.
This surge in regenerative medicine market advancements has transformed therapeutic approaches and given rise to a burgeoning global market. The availability of diverse treatments, from orthopaedics to cosmetic procedures, reflects the industry’s substantial growth, fostering both innovation and accessibility worldwide.
- With the introduction of Regenerative Medicine (RM), the beachside progress has been remarkable, reflected in over 280,000 PubMed search results related to regeneration. Scientists across disciplines, including cell/molecular biologists, engineers and clinicians, have driven a shift from treatment-focused to curative therapies.
- Over the past 20 years, the field’s influence, capabilities and research output have surged, making Tissue Engineering a prominent focus for the scientific community.
- As of January 2021, data from the United States Organ Procurement and Transplantation Network reveals 118,618 patients awaiting transplantation. Despite approximately 100 daily transplants, a new patient is added to the list every 9 minutes, underscoring the urgency and significance of advances in Tissue Engineering and Regenerative Medicine.
The Role of Increasing Prevalence of Brain Injury
The increasing prevalence of brain injuries has led to a growing demand for effective treatment options, driving the global regenerative medicine market. Regenerative medicine offers promising solutions for neurological disorders, including brain injuries, by harnessing the body’s natural healing mechanisms. Brain injuries often result in long-term consequences and conventional treatments have limitations. Regenerative medicine, focusing on repairing or replacing damaged tissues, addresses the unmet medical needs in neurology.
Stem cell therapies, a key component of regenerative medicine, have shown potential in promoting neural regeneration and functional recovery after brain injuries. This has fueled research and development efforts, contributing to the growth of the regenerative medicine market. Tissue engineering, another facet of regenerative medicine, involves creating artificial tissues to replace or support damaged ones. In the context of brain injuries, this technology holds promise for rebuilding neural circuits and restoring functionality.
Regenerative medicine market approaches not only aim to repair damage but also focus on providing neuroprotective effects, preventing further deterioration. This holistic approach aligns with the complex nature of brain injuries, making regenerative therapies more attractive. Ongoing clinical trials and the potential approval of regenerative medicine treatments for brain injuries have generated considerable interest from investors and pharmaceutical companies. Positive outcomes in trials can significantly impact market dynamics.
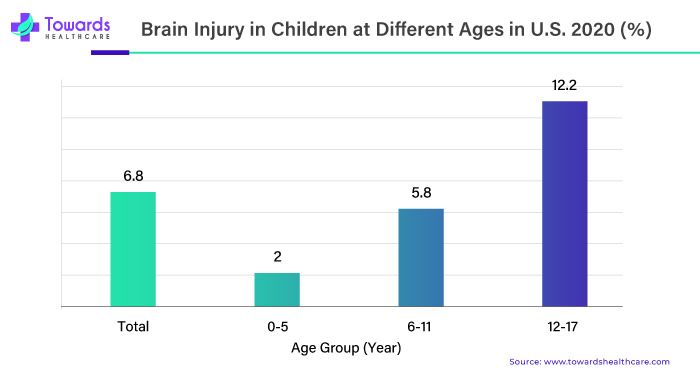
According to age, the percentage of children who had ever symptoms of a concussion or brain injury varied. In 2020, 6.8% of children had experienced symptoms of a concussion or brain injury in their lifetime. The percentage of children who had ever had symptoms of a concussion or brain injury with age went from 2% in children aged five years and under to 12.2% in children aged 12-17.
 For Instance,
For Instance,
- In 2019, Traumatic Brain Injury (TBI) witnessed 27.16 million new cases globally, with 48.99 million prevalent cases and 7.08 million Years Lived with Disability (YLDs). Spinal Cord Injury (SCI) had 0.91 million new cases, 20.64 million prevalent cases, and 6.20 million YLDs. The prevalence of trauma injuries has surged, driving the adoption of regenerative medicine and organ transplantation. A notable increase in accidents, burns, and other trauma injuries has propelled the regenerative medicine market.
- The Centers for Disease Control and Prevention reported 223,135 TBI-related hospitalizations in 2019 and 64,362 TBI-related deaths in 2020, averaging more than 611 hospitalizations and 176 deaths daily in the U.S. This rise in accident trauma cases globally has heightened the demand for regenerative medicines.
Several companies are expanding their presence in the trauma injuries regenerative medicine market. Although tissue engineering and regenerative medicine are currently not dominant in trauma injuries, advancements in accidental care technologies are expected to fuel rapid market growth in the forecast period, contributing to the global upsurge. Additionally, Collaborations between academic institutions, research organizations and industry players have accelerated the pace of discovery in regenerative medicine market.
This collaborative effort contributes to a robust pipeline of potential treatments for brain injuries. Governments and private sectors increasingly invest in healthcare, including regenerative medicine research. This financial support has a direct impact on the growth of the regenerative medicine market, including its application in treating brain injuries.
Growing awareness of regenerative medicine’s potential benefits for brain injuries, coupled with patient advocacy, has influenced healthcare policies and increased funding for related research, positively impacting market expansion; the increasing prevalence of brain injuries has catalyzed a surge in research and development within the regenerative medicine market. This, coupled with advancements in stem cell therapy, tissue engineering and neuroprotective approaches, has positioned regenerative medicine as a key player in addressing global brain injuries complex challenges.
Stem Cell Therapy, A Revolutionary Application in Regenerative Medicine, Creates Growth Opportunities
Stem cell therapy is a groundbreaking application within regenerative medicine market, showcasing its real-world impact. This innovative approach utilizes stem cells or their derivatives to foster the healing response of damaged, dysfunctional or injured tissues. Representing a significant leap forward, it has the potential to revolutionize organ transplantation by employing cells instead of relying on limited donor organ availability.
In laboratories, scientists nurture and guide stem cells to specialize in specific cell types, such as blood cells, heart muscle or nerve cells. Once specialized, these cells can be transplanted into individuals, harnessing the remarkable capability of stem cells to generate all tissues in the human body. This presents immense possibilities for future applications in tissue repair and regeneration.
Additionally, this transformative technology isn’t just a medical breakthrough; it’s a driving force behind the surge in the global market. As the demand for advanced healthcare solutions rises, the market for stem cell therapy is experiencing a substantial increase, reflecting the growing recognition of its potential and effectiveness on a global scale.
For Instance,
- In 2021, the National Institutes of Health (NIH) identified over 2,700 US clinics offering questionable stem cell treatments. Meanwhile, the FDA greenlit groundbreaking treatments, Casgevy and Lyfgenia, marking the first cell-based gene therapies for sickle cell disease in patients aged 12 and older. Casgevy, among them, stands out as the inaugural FDA-approved treatment utilizing innovative genome editing technology, signifying a significant leap in gene therapy.
- In 2022, robust governmental backing for stem cell research propelled the growth of stem cell companies globally. Notably, the NIH allocated $3.7 billion for stem cell research, while various countries initiated programs to support these companies. The UK, for example, established a £30 million ($37 million) fund to aid stem cell firms in bringing novel therapies to market.
In 2022, stem cell companies made the following specific advances:
Clinical studies on stem cell therapies:
- In 2022, various stem cell therapies will be put into clinical trials. Bluebird Bio, for example, has begun phase 3 clinical treatment trials using stem cells for sickle cell disease.
Regulatory clearance for stem cell companies:
- In 2022, these are but a handful of the numerous advancements the stem cell sector saw in 2022. This rise is primarily due to the growing support from the government, which is expected to encourage the creation of new stem cell medicines and the expansion of stem cell companies in the years to come.
Regulatory Hurdles Restrain the Regenerative Medicine Market Growth
Experts we spoke to highlighted various challenges in regulating regenerative medicine. One key issue is the limited access to regulatory expertise, particularly for smaller entities like start-ups and academic institutes needing more dedicated regulatory departments. This deficiency can hinder the entire product development process, including the crucial stage of submitting a product for FDA review. To address this, a suggested policy option involves consistent support for measurement science research, which is essential for developing regenerative medicine market technologies.
The proposed implementation approaches include government agencies like NIST and the FDA allocating specific funding for measurement science research. Similarly, industry stakeholders are encouraged to invest more in measurement science initiatives. However, potential challenges arise, such as the risk of redirecting resources from other emerging technologies and the reluctance of private industry to invest without a swift return.
Additionally, the absence of standardized measurements may lead to technology failures during development or regulatory review. Companies might incur higher costs to meet safety requirements, and reluctance to adapt existing processes could pose further obstacles. In the complex regulatory landscape, the lack of regulatory expertise may result in delays and resource wastage for companies, potentially generating data that does not meet FDA standards. Access to knowledgeable regulatory experts and opportunities to interact with FDA reviewers are crucial for overcoming these challenges.
Additionally, the regenerative medicine market faces additional hurdles from regulatory and ethical considerations. These factors create complexities that impact product development, approval processes and overall market dynamics. Balancing regulatory compliance and ethical standards is essential for fostering innovation and ensuring the success of regenerative medicine market in the technologies.
The Regenerative Medicine Market is Growing Significantly due to Applications in the Musculoskeletal System
The regenerative medicine market is witnessing significant growth, driven by applications in the musculoskeletal system. This field focuses on developing innovative therapies to repair, replace or regenerate damaged tissues within the musculoskeletal system, which includes bones, muscles, tendons, ligaments and joints.
Regenerative medicine market is making strides in treating orthopaedic conditions such as osteoarthritis, tendinopathies and ligament injuries. Stem cell therapies and growth factor treatments are being explored to promote tissue repair and alleviate symptoms associated with these conditions. Cartilage injuries and degeneration are common challenges in orthopaedics.
Regenerative approaches, including autologous chondrocyte implantation (ACI) and scaffold-based techniques, are being researched to restore cartilage function and delay the progression of osteoarthritis. Mesenchymal stem cells (MSCs) have shown promise in musculoskeletal regenerative applications. These cells can differentiate into musculoskeletal tissues, including bone, cartilage and muscle. Stem cell therapies aim to enhance the body’s natural healing processes.
Advances in biomaterials contribute to the development of biological implants that mimic the properties of musculoskeletal tissues. Often combined with cells or growth factors, these implants offer potential solutions for joint replacements and soft tissue reconstruction. For conditions such as fractures, non-unions or bone defects, regenerative medicine market explores techniques like bone grafts, growth factors and bone tissue engineering to stimulate new bone formation and improve healing outcomes.
The intersection of regenerative medicine and musculoskeletal applications holds promise for improving patient outcomes, reducing the need for traditional surgical interventions and addressing unmet medical needs in orthopaedics.
Geographical Landscape
Geographically, North America dominates the regenerative medicine market due to its high prevalence of chronic diseases and sophisticated healthcare framework. Conversely, Asia-Pacific is expected to increase as healthcare expenditure rises and major manufacturers invest in new product launches. The North American regenerative medicine market is expanding. It is expected to have high potential in the future, as most therapies are in the third phase of clinical trials. This demonstrates the involvement of companies in the commercialization of regenerative medicine market products. Favourable regulatory and reimbursement policies for tissue engineering illustrate the interest of government authorities in meeting the growing demand for regenerative products.
The stringent regulatory policies governing stem cell technologies have posed significant challenges to adopting embryonic stem cells, owing to ethical concerns and controversies surrounding their source, thereby limiting market growth. These challenges are expected to be addressed as companies and research institutes develop novel therapies that will replace embryonic stem cells in various applications.
Furthermore, technological advancements for tissue and organ transplantation, emerging technologies and a focus on stem cells contribute to the market’s growth. Among North American countries, the United States is the most significant contributor to market growth, Canada provides a productive environment, and Mexico has a well-established research and development infrastructure.
The Asia-Pacific includes China, Japan, India, Indonesia, Myanmar, Thailand and Australia countries. It has a significant influence on the market due to its diverse culture and demographics. The high incidence rate of unintentional trauma injuries has accelerated the growth of the regenerative medicine market.
Global players have focused on providing patients with cost-effective therapies and adopting novel stem cell-based technologies that can be used to develop disease-treatment products. This is primarily due to an increase in the incidence of injuries in the growing senior population. Lack of awareness about regenerative medicine market and stringent growth regulations stifle market growth.
Japan has the most influence on pharmaceutical and biotechnology markets in the Asia Pacific, while China has the fastest-growing economy. Furthermore, due to the increase in geriatric patients, Australia’s healthcare costs are higher than those of the other Asian Pacific countries. The rest of the region offers substantial growth opportunities to market players.
Competitive Landscape
The regenerative medicine market is dynamic, with key players driving innovation and growth. Companies like Medtronic, Thermo Fischer Scientific and Novartis are prominent, focusing on stem cell therapies, tissue engineering, and gene therapies. Startups such as Bluebird Bio and CRISPR Therapeutics contribute to the competitive landscape, emphasizing gene editing advancement. Collaboration between pharmaceutical giants and biotech firms and increasing research initiatives shape the evolving competitive scenario in regenerative medicine.
Market Players
- Medtronic
- Stryker
- Zimmer Biomet
- Pfizer Inc
- Novartis AG
- Bristol-Myers Squibb
- Vertex Pharmaceuticals Incorporated
- Gamida Cell
- Ferring B.V.
- Syngene International Limited
Market Segments
By Product
- Cell-Based Products
- Tissue Engineered Products
- Gene Therapy Vectors
- Others
By Application
- Wound Care
- Musculoskeletal
- Ophthalmology
- Oncology
- Cardiovascular
- Dermatology
- Neurology
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa