Retinitis Pigmentosa Market Overview Growth and Emerging Opportunities
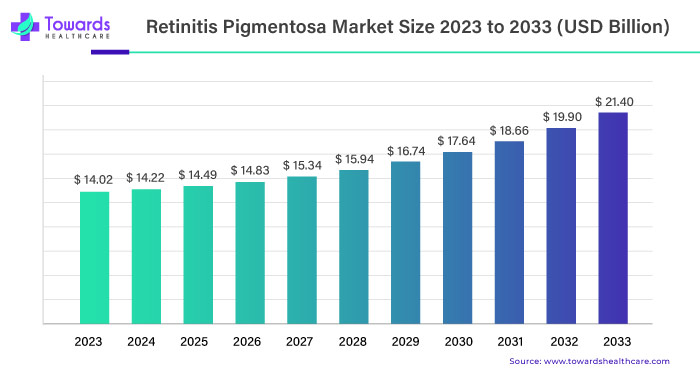
The global retinitis pigmentosa (RP) market was valued at USD 14.02 billion in 2023 and is expected to grow significantly, reaching USD 21.40 billion by 2033. This growth represents a compound annual growth rate (CAGR) of 6.2% from 2023 to 2033.
In 2023, autosomal recessive RP was the dominant form, accounting for 65% of the market share. However, gene therapy is gaining attention, with a promising 23% market share, offering new hope for many patients.
Currently, North America holds the largest share of the RP market, but Asia Pacific is experiencing the fastest growth, potentially reshaping the market’s future dynamics.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5114
In 2022, it was estimated that between 82,500 and 110,000 people in the United States were living with retinitis pigmentosa (RP), highlighting the increasing need for new treatments, such as gene therapy and retinal implants.
Retinitis pigmentosa refers to a group of genetic eye disorders that affect the retina, the part of the eye responsible for vision. The condition causes the gradual deterioration of photoreceptor cells, which are essential for seeing in different lighting conditions and maintaining clear vision. As RP progresses, people often experience difficulty seeing in low-light or at night, a loss of peripheral vision, and, in some cases, complete blindness.
There are two main types of retinitis pigmentosa: non-syndromic and syndromic. Non-syndromic RP, which accounts for 70-80% of cases, does not involve other systemic health issues. On the other hand, syndromic RP, which makes up 20-30% of cases, is linked to additional health conditions outside of the eyes. Non-syndromic RP is caused by mutations in about 80 different genes, with 20% of cases being autosomal recessive, 10-20% autosomal dominant (AD), and 10% X-linked recessive.
A 2021 study by the National Institutes of Health, conducted in Spain (which has one of the largest patient populations for inherited retinal diseases), also found new insights into X-linked retinitis pigmentosa (XLRP). While it was previously thought that only males were affected by XLRP, it is now recognized that female carriers can also show symptoms, ranging from mild to severe retinal disease. The remaining cases of RP are considered “sporadic,” meaning no clear inheritance pattern or molecular cause has been identified.
Recent Developments in Retinitis Pigmentosa Treatment
In January 2022, 4D Molecular Therapeutics announced that the FDA had granted special status to 4D-125, a promising treatment for X-linked retinitis pigmentosa (XLRP). This therapy is designed to deliver a functional version of the RPGR gene directly to the retina’s light-sensitive cells and is formulated for easy administration into the eye.
Also in 2022, Bionic Vision Technologies (BVT) received Breakthrough Device designation from the FDA for their Bionic Eye System. This recognition marks a major step forward in the field of bionic vision technology, with the system offering hope for people with severe vision impairments.
In the Netherlands, a patient underwent the first procedure to receive the Prima System, a bionic eye implant developed by Pixium Vision in France. This innovative implant holds promise for people suffering from geographic atrophy (GA), a form of dry age-related macular degeneration (AMD), offering the potential for partial vision restoration.
Key Companies in the Retinitis Pigmentosa Market
- Novartis: A global healthcare company involved in developing treatments for a range of eye diseases, including retinitis pigmentosa.
- Roche (Spark Therapeutics Inc.): Known for its gene therapy solutions, Spark Therapeutics is making strides in addressing genetic eye diseases like retinitis pigmentosa.
- MeiraDx: A biotechnology firm focused on the development of diagnostic and therapeutic products for various retinal conditions.
- Astellas Pharma Inc.: A leading pharmaceutical company investing in gene therapy research and development for retinal diseases.
- Clino Corporation: A biotechnology company focused on rare genetic diseases, including retinitis pigmentosa.
- Gensight Biologics: A clinical-stage biotechnology company working on gene therapies aimed at treating retinal degenerative diseases like RP.
- InFlectis BioScience: A company focused on developing therapies for ocular diseases, including retinitis pigmentosa, with a focus on gene therapy.
- Bausch Health Companies Inc.: A global healthcare company with a growing portfolio of treatments for eye conditions, including those affecting the retina.
- Adverum Biotechnologies: A biotechnology company developing gene therapies for retinal diseases, including a potential treatment for RP.
- ReGenx Biosciences: A biotechnology company specializing in gene therapies for genetic disorders, including retinitis pigmentosa.
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/retinitis-pigmentosa-market-sizing
Access exclusive insight now @ https://www.towardshealthcare.com/price/5114
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare