Biosimilars Market Development and Impact on Healthcare Growth and Innovations
Table of Contents
ToggleBiosimilars Market Growth, Key Players and Driving Factors
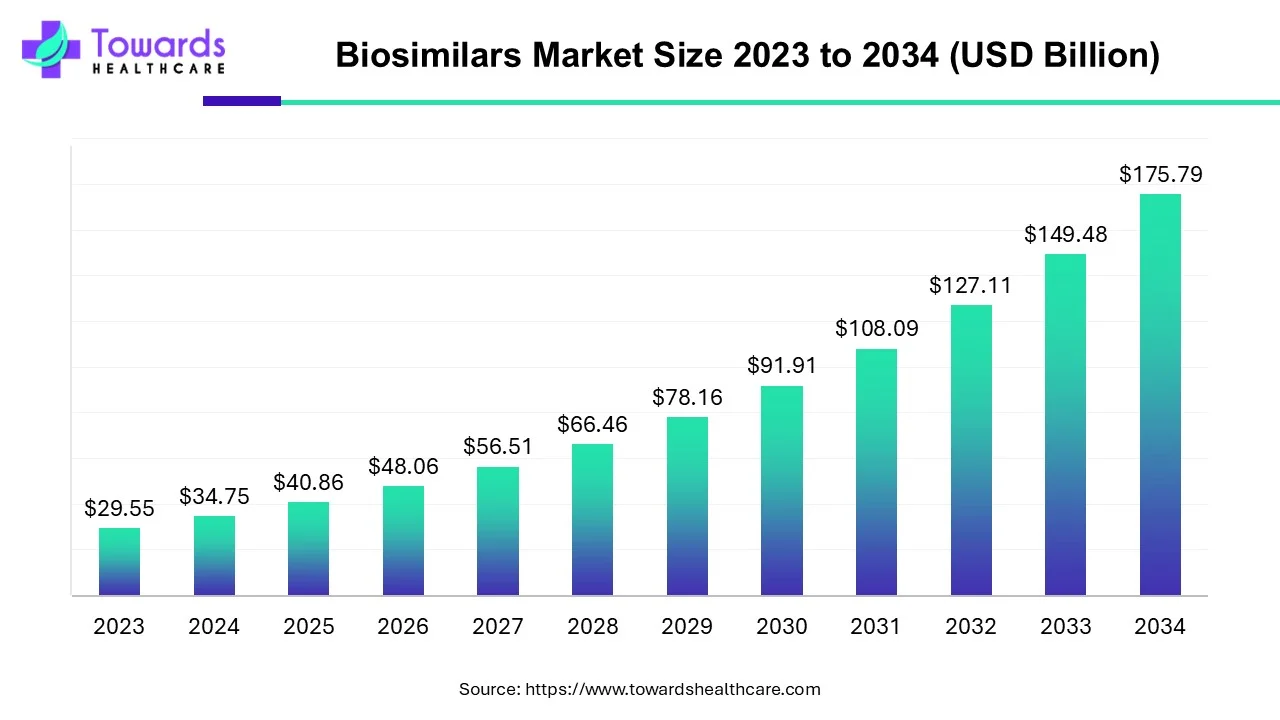
The global biosimilars market is projected to expand from USD 34.75 billion in 2024 to approximately USD 175.79 billion by 2034, growing at a compound annual growth rate (CAGR) of 17.6% from 2025 to 2034. This growth is primarily driven by the increasing prevalence of cancer and the cost-effectiveness of biosimilars.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5035
Biosimilars Market Trends
In November 2024, Aurobindo Pharma’s subsidiary, CuraTeQ Biologics, received a GMP certificate from the European Medicines Agency (EMA) for its biosimilar manufacturing facility in Hyderabad. This facility will produce high-quality biosimilars for patients worldwide. In August 2024, Biocon Biologics partnered with Janssen to launch a biosimilar for autoimmune diseases in Europe, the U.S., Canada, and Japan. Also, in October 2024, Teva Pharmaceuticals and mAbxience expanded their collaboration for developing an anti-PD1 oncology biosimilar.
Biosimilars are biological products that are very similar to existing FDA-approved reference products, offering comparable efficacy, safety, and quality after rigorous clinical testing. While not identical to their reference drugs, biosimilars are derived from living cells, which may cause minor differences in manufacturing processes. Despite this, they deliver the same clinical outcomes as their reference products.
Biosimilars are much more affordable than their reference biologics, offering significant cost savings to patients, healthcare providers, and insurers. As more biologic drugs lose patent protection, the demand for affordable treatments is increasing, fueling the rapid growth of the biosimilars market. The approval process for biosimilars is highly regulated and complex, as manufacturers must prove the products’ similarity to the reference drugs through extensive clinical trials.
These medications are used to treat chronic conditions like rheumatoid arthritis, anemia, certain cancers, and inflammatory diseases. Biosimilars are usually priced 10 to 40% less than their reference products, making them more accessible to patients and improving adherence, which leads to better health outcomes. They are also increasing market competition, driving down prices and offering consumers savings. It is projected that FDA-approved biosimilars could save up to $250 billion in the first ten years after their market entry.
Impact of AI in the Biosimilar Market
Artificial intelligence (AI) is revolutionizing biosimilar development by improving the speed and quality of research and production. AI helps researchers understand complex protein structures, making biosimilar development easier. Machine learning (ML) algorithms are used to analyze biosimilar properties, monitor particle consistency, and optimize manufacturing processes to enhance accuracy and reduce errors. Predictive analytics powered by AI can also identify potential production or supply chain issues, helping reduce costs and improve efficiency. AI tools are also capable of analyzing large datasets from clinical studies, enabling better decision-making in biosimilar development.
The Role of Biosimilars in Cancer Treatment
Biosimilars are gaining recognition as a cost-effective alternative to expensive biologic drugs for cancer treatment. Biologics, which are complex drugs derived from living organisms, are widely used in oncology but come at a high cost, limiting access to treatment for many patients, particularly in low- and middle-income countries.
Biosimilars, which are highly similar to their reference biologics, offer an affordable option without compromising on clinical efficacy or safety. Several biosimilars, such as those for monoclonal antibodies used in breast cancer, colorectal cancer, and non-Hodgkin’s lymphoma, have already been approved. Additionally, biosimilars of granulocyte colony-stimulating factors (G-CSF) are used to treat neutropenia, a side effect of chemotherapy. Oncology-related biosimilars contributed around 24.29% of the market share in 2022 and are expected to grow at the fastest rate in the coming years.
Biosimilars can increase patient access to life-saving cancer treatments, especially in developing countries, while also reducing the financial burden on healthcare systems. Despite concerns about their safety and long-term efficacy, extensive clinical trials and regulatory testing ensure biosimilars are safe for use. Ongoing research and monitoring will be essential to confirm their long-term effectiveness.
Segment Insights
- Recombinant Non-Glycosylated Proteins Dominating the Market
In 2024, recombinant non-glycosylated proteins held the largest share in the biosimilars market. These proteins, such as growth hormones, insulin, and interferon, are produced through recombinant techniques without glycosylation. They are used to treat chronic conditions like cancer and diabetes, driving their growth. - Recombinant Glycosylated Proteins: Fastest-Growing Segment
The recombinant glycosylated proteins segment is expected to grow rapidly. These proteins are modified with sugar molecules after translation and include insulin, growth factors, and monoclonal antibodies. Advancements in technology and increased R&D investments are fueling the growth of this segment.
Recent Developments in the Biosimilars Market
In April 2024, Alvotech and Teva Pharmaceuticals received approval from the U.S. FDA for their biosimilar product, SELARSDI (ustekinumab-aekn) injection. This biosimilar is designed to treat psoriasis and is intended to compete with the branded drug Stelara. Teva will exclusively handle the commercialization of SELARSDI in the U.S.
In another significant move, Lek, a pharmaceutical company from Slovenia, began constructing a new biosimilar development center in Ljubljana. The $90 million investment is aimed at expanding their capabilities in biosimilar production.
Leading Companies in the Biosimilars Market
- Novartis
- Synthon Pharmaceuticals, Inc.
- Teva Pharmaceutical Industries Ltd.
- LG Life Sciences
- Celltrion
- Biocon
- Hospira
- Merck Serono
- Biogen Idec, Inc.
- Genentech
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/biosimilars-market
Access exclusive insight now @ https://www.towardshealthcare.com/price/5035
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare