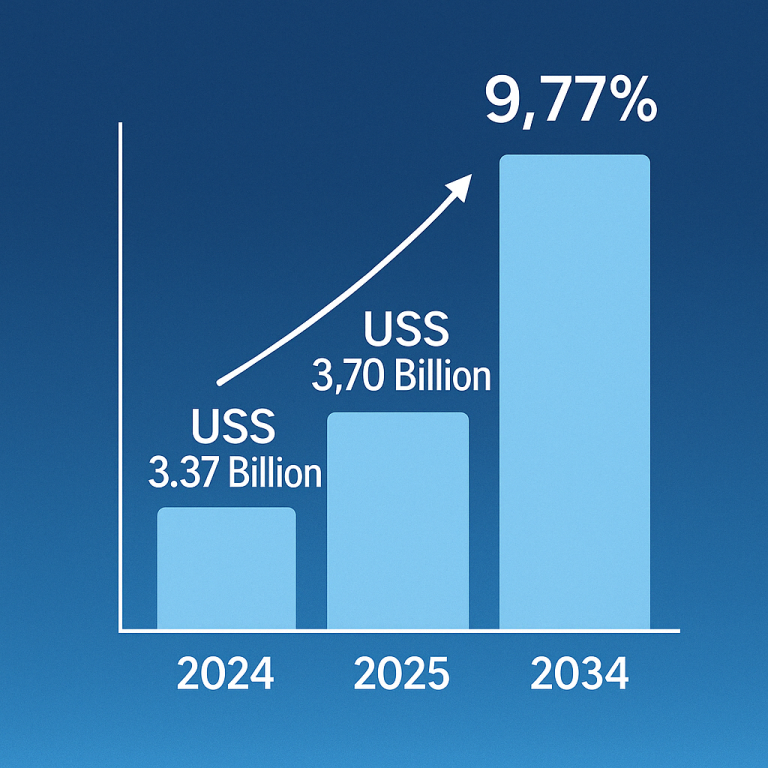
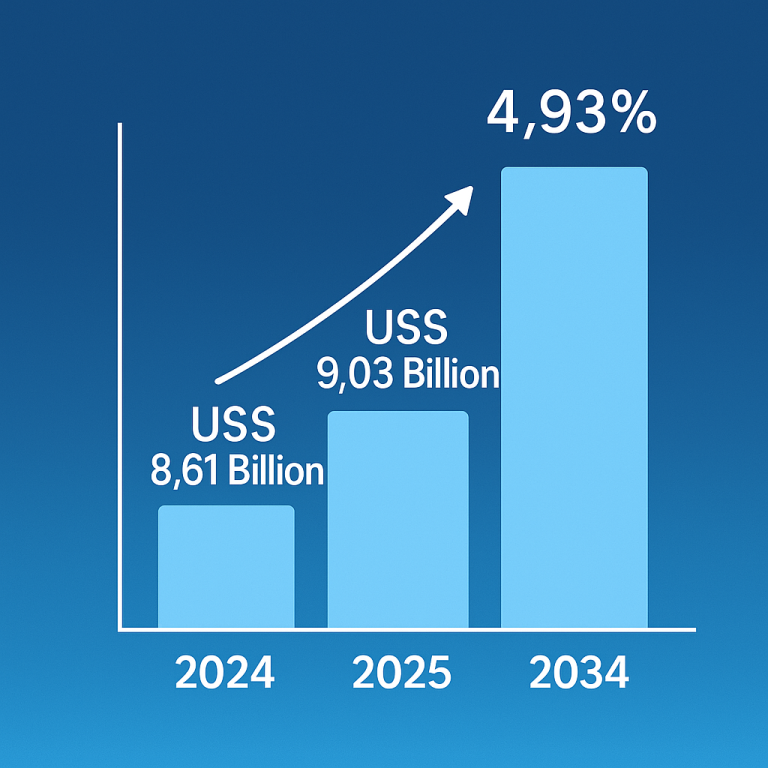
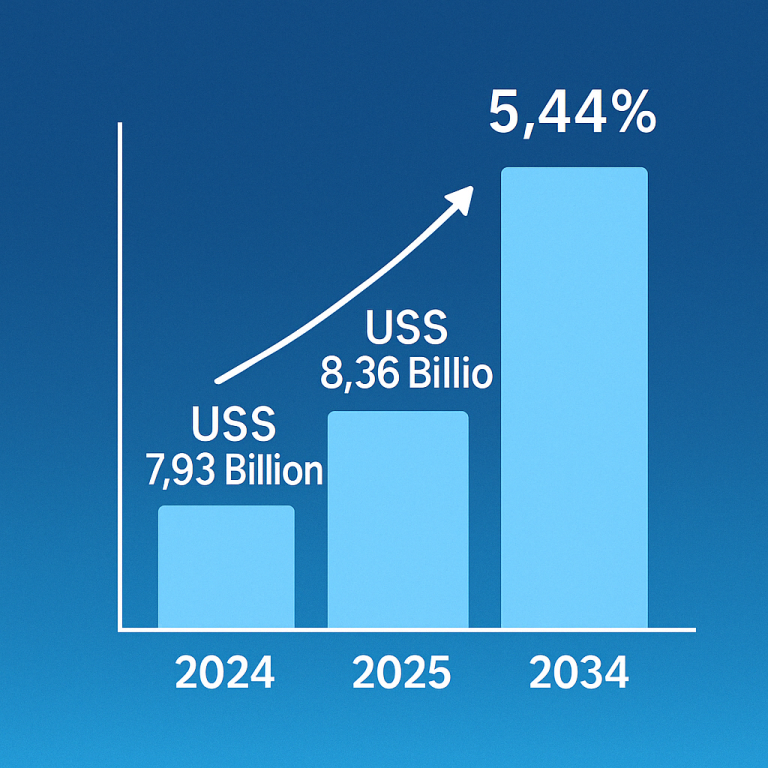
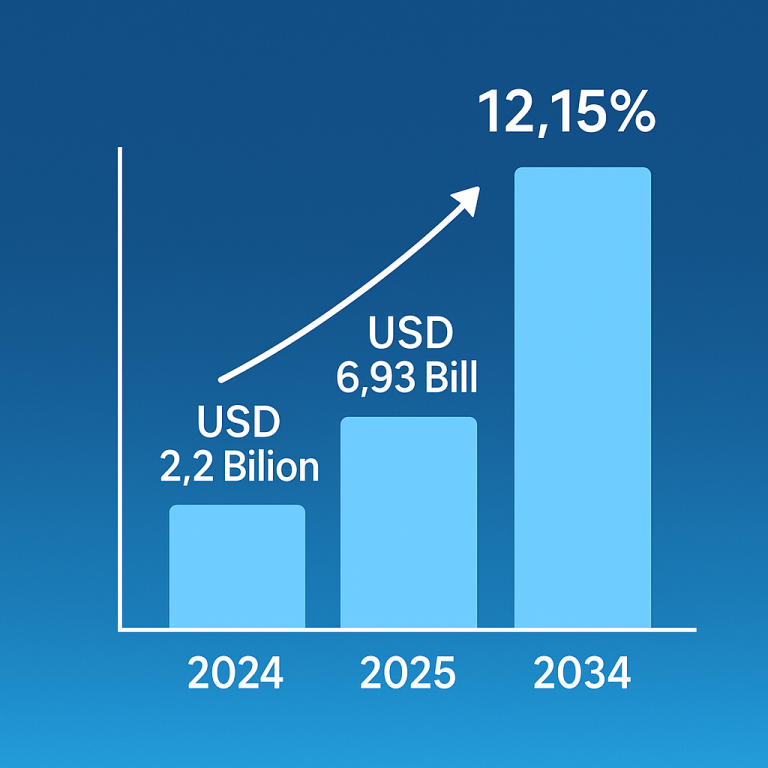
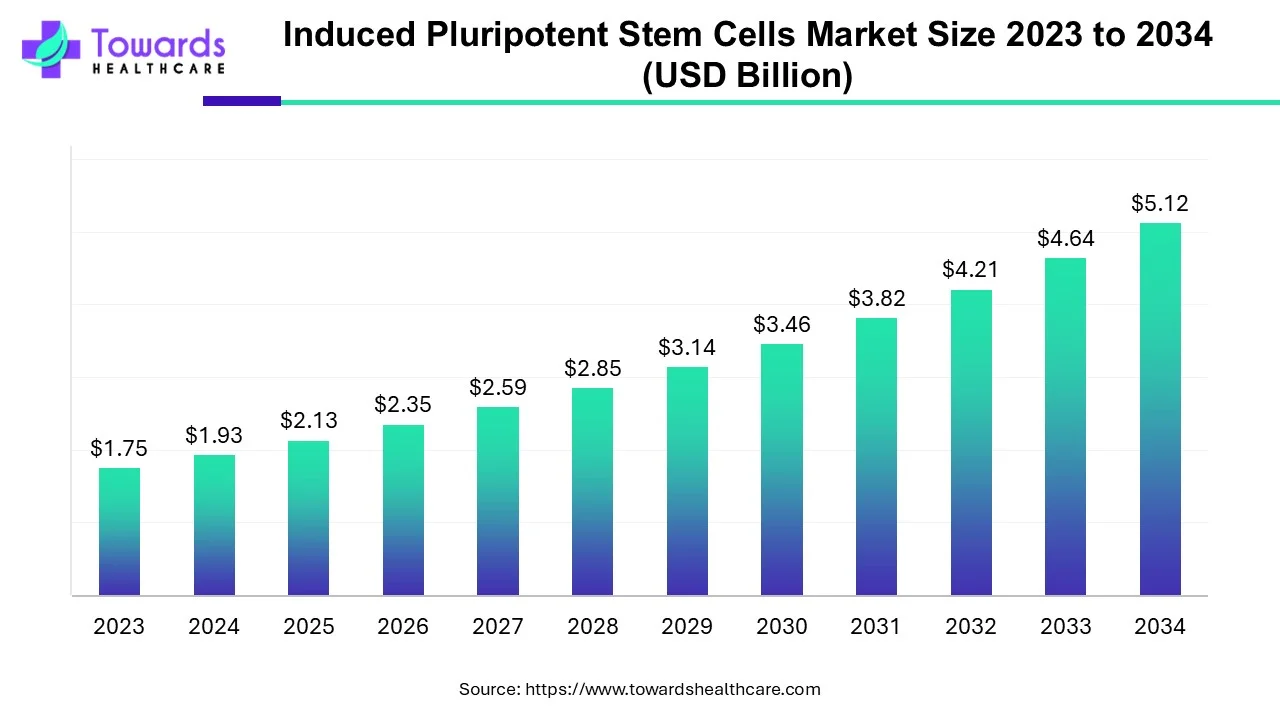
The global induced pluripotent stem cells market size is calculated at USD 1.93 billion in 2024, grew to USD 2.13 billion in 2025, and is projected to reach around USD 5.12 billion by 2034. The market is expanding at a CAGR of 10.25% between 2024 and 2034. The rising prevalence of chronic disorders, growing demand for regenerative medicine, and technological advancements drive the market.
Get All the Details in Our Solution – Download Brochure @ https://www.towardshealthcare.com/download-brochure/5373
Growing Demand for Regenerative Medicine
The demand for regenerative medicines has been increasing for many decades due to the rising incidences of chronic disorders. Regenerative medicines have been extensively studied for treating conditions such as cancer, diabetes, neurological disorders, heart failure, osteoarthritis, and other ailments. Novel applications for regenerative medicine are continuously evaluated, offering groundbreaking possibilities for healthcare advancements.
Regenerative medicine enables scientists to grow tissues and organs in laboratories, allowing safe implantation when the body is unable to heal itself. The increasing research and development activities related to regenerative medicine significantly boost the growth of the induced pluripotent stem cells (iPSCs) market. This has led to a surge in clinical trials examining the effects of iPSCs on humans. As of November 2024, approximately 40 clinical trials related to iPSCs have been registered on clinicaltrials.gov. Additionally, the demand for iPSCs is rising due to favorable government support, increased investments, and growing collaborations in the field.
Manufacturing Challenges
One of the major challenges facing the induced pluripotent stem cells market is the high manufacturing cost of iPSC technology. The cost of generating and expanding an iPSC line, along with the necessary tests to assess its pluripotency and safety, ranges from $10,000 to $20,000 on average. For clinical applications, the cost of iPSC cell lines can escalate to $1 million, posing a significant financial burden on research institutions, particularly in low- and middle-income countries (LMICs).
Additionally, the production of iPSCs is a time-intensive process, taking approximately four to six months. These high costs and lengthy production timelines create challenges in the widespread adoption and accessibility of iPSC technology, ultimately restricting market growth.
Latest Advancements in iPSC Technology
The growing demand for iPSCs has fueled the development of novel advancements in iPSC technology. Researchers are continually exploring expanded applications of iPSCs and devising innovative extraction methods. Notably, small-molecule compounds have been developed to generate iPSCs from mouse cells, demonstrating the potential for chemical reprogramming as a future strategy for different somatic cells.
Furthermore, iPSCs are now being used to create disease models that help scientists study the mechanisms of human genetic disorders and test novel drug therapies. iPSCs hold immense potential in personalized medicine by providing patient-specific models for customized treatments.
Additionally, iPSC technology is increasingly being integrated with genome editing, 3D cell culture systems, and other emerging techniques to advance research. The use of iPSCs in medicine holds a promising future, with potential breakthroughs in precision medicine, novel treatment developments, and more sophisticated research models.
Recent advancements further highlight the potential of iPSC technology:
- In September 2024, Chinese researchers demonstrated the ability of chemically induced pluripotent stem cells to reverse type-1 diabetes using patient-derived cells. Pluripotent stem cells were chemically reprogrammed and converted into insulin-producing cells, showcasing a potential therapeutic approach.
- In August 2024, researchers from the Centre for Genomic Regulation developed a treatment utilizing iPSCs to accelerate their production and quality in mice. This advancement aims to improve disease modeling and drug testing, particularly for individuals with two X chromosomes and people with Klinefelter Syndrome.
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/induced-pluripotent-stem-cells-market-sizing
Invest in Our Premium Strategic Solution @ https://www.towardshealthcare.com/price/5373
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare