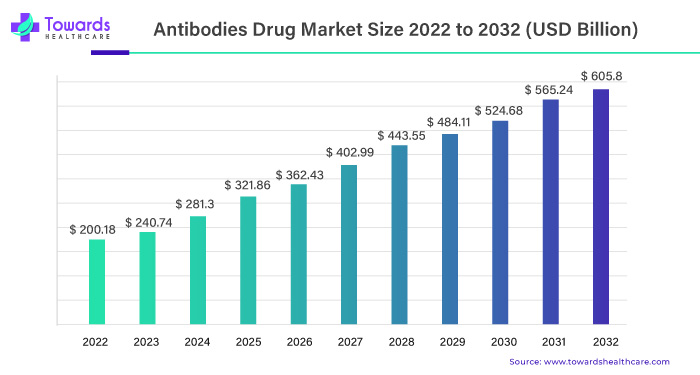
The global antibodies drug market has witnessed a remarkable ascent, reaching a substantial USD 200.18 billion in 2022. Projections now anticipate an even more impressive surge, with expectations set to exceed USD 605.8 billion by 2032. This phenomenal growth is propelled by a robust Compound Annual Growth Rate (CAGR) of 10.8% between 2023 and 2032, showcasing the industry’s resilience and potential.
Driving Forces behind the Surge
Monoclonal Antibodies Drug Market Production Boom
The catalyst behind this exponential growth lies in the surge of monoclonal antibodies production. As technological advancements continue to enhance the manufacturing processes, the resulting increase in monoclonal antibodies contributes significantly to the escalating demand for antibodies drug market.
Forecasting the Future
Projections for 2032
With the current trajectory, the antibodies drug market is poised for an extraordinary expansion, surpassing the USD 605.8 billion mark by 2032. The robust CAGR of 10.8% forecasted between 2023 and 2032 is indicative of sustained and substantial growth, highlighting the market’s ability to meet the escalating demand for innovative healthcare solutions.
Implications for Antibodies Drug Market Healthcare
The surge in the antibodies drug market signifies a paradigm shift in healthcare dynamics. This growth is not merely a numerical milestone but represents an increased reliance on advanced therapeutic solutions. Monoclonal antibodies, with their precision and efficacy, are becoming pivotal players in addressing a myriad of medical conditions, contributing to the industry’s transformation.
Monoclonal Antibodies (mAbs), which seem to be antibody preparations designed to bind to a single target, have shown potential in the fight against cancer as well as autoimmune diseases.
Monoclonal antibodies are protein-based therapies or drugs that are increasingly being used to treat chronic diseases. This blog examines the global antibodies drug market products and provides an update on their applications in various disease areas. The overall antibodies drug market products is divided into four major application areas: autoimmune diseases, solid tumors, lymphoma, and leukemia, as well as other diseases such as asthma, osteoporosis, and cardiovascular disease.
The FDA has approved more than 100 antibody products, and biologics account for roughly one-fifth of all new drug approvals each year. The presence of a healthy antibody therapy pipeline increases the company’s chances of achieving higher market revenue. As a result of the high demand for antibody therapy, a surge in the development of new antibody products is expected to drive the antibody therapy market share.
Which Was The First Antibody-Drug?
Orthoclone OKT3 (muromonab-CD3) was the first licensed monoclonal antibody, approved in 1986 for use in preventing kidney transplant rejection.
It is a mouse monoclonal IgG2a antibody with the cognate antigen CD3. It works by binding and inhibiting the effects of CD3 on T-lymphocytes. However, due to reported side effects, its use was restricted to acute cases (e.g. human anti-mouse antibody response)
Which are the Influencers Fueling The Antibodies Drug Market Growth
Immunotherapy’s Expanding Applications Are Expected To Drive Up Demand For Antibodies Drug Market:
Targeted therapies are intended to attack cancer cells using antibodies and chemotherapy. They are based on the idea that cancer cells have abnormal versions of certain proteins known as receptors that exist in normal cells but not in cancer cells. Antibodies against these receptors may be used in these targeted therapies. Immunotherapy is a type of cancer treatment that employs the immune system to combat the disease. It is also known as biotherapy. This type of treatment can be used alone or in conjunction with other therapies. Immunotherapy is classified as a biological therapy. Biological therapy is a type of cancer treatment that uses compounds derived from living organisms.
Rising cancer cases around the world are expected to boost demand for antibody therapy. According to the Global Cancer Observatory (GLOBOCAN), approximately 19.3 million new cancer cases will be reported in 2020, with approximately 10 million associated deaths. according to the World Health Organization (WHO), cancer is the first/second leading cause of death in many countries, reducing life expectancy.
Cancer’s increasing prevalence and high mortality rate have increased the demand for effective and targeted therapies such as antibody therapy. Recently, antibody-based treatments have advanced technologically by targeting tumor antigens and enhancing immune cells’ anti-tumor capabilities. Furthermore, high product advancement and technologies for cancer treatment are expected to drive the antibodies drug market therapy growth.
Demand For Low-Cost Bio-Similar Monoclonal Antibodies Drug Market Is Growing
The popularity of the cost-effective bio-similar monoclonal antibodies drug market is rapidly increasing, which boosts monoclonal antibodies market growth. The goal of bio-similar antibodies is to control rising healthcare costs and to deal with economic pressure from patients and governments to reduce medication costs and improve treatment approaches. A bio-similar monoclonal antibody is 20%-25% less expensive than the original biologic drug. The number of clinical trials for a biosimilar is less than that of the original biologic drug, which explains why biosimilar drugs are less expensive.
Hurdles Faced During The Antibodies Drug Market Expansion
The Approval Process Required Various Stringent Regulatory Policies Which Might Hinder The Antibodies Drug Market Growth
The long, as well as strict regulations for approval and launch, will be a major hurdle faced by the Antibodies drug market. The failure to meet the stringent regulations and results had led to the withdrawal of various clinical studies by the regulatory authorities.
The Functional Disadvantages Of Therapeutic Antibodies Are Impeding Antibodies Drug Market Growth
Certain functional disadvantages of therapeutic antibodies, such as insufficient pharmacokinetics and tissue accessibility, as well as impaired interactions with the immune system, have been identified, and these deficiencies indicate areas where more research is needed. When monoclonal antibodies were first developed, they encountered serious problems when used as therapeutics. The first monoclonal antibodies were murine molecules that were recognized as foreign when injected into patients, causing the immune system to remove them. Furthermore, in order to be effective, antibodies frequently need to interact with immune system components such as receptors on effector cells or the complement cascade.
Open Doors For The Market Growth
Increasing Investment In The Development Of Novel Therapeutics
Monoclonal antibodies are gaining popularity as a safe and effective treatment for a variety of chronic diseases. Due to the success of drugs such as Remicade, Avastin, Rituxan, and Herceptin, demand for these products is rapidly increasing. Furthermore, manufacturers are engaged in the development of new drugs and product launches, which will accelerate market growth in the near future.
Market Challenge
Monoclonal Antibodies Are Expensive
The mAbs are very expensive. The low financial capabilities of the low and middle-income groups in developing and underdeveloped countries, as well as a lack of proper access to healthcare facilities, pose a significant challenge to market players and may impede the growth of the global monoclonal antibodies market.
Ill Effects Of COVID-19 On The Antibodies Drug Market Expansion
COVID-19 had a significant impact on the global economy, including all nations, by interrupting the operations of all sectors, regardless of size. The rising prevalence of COVID-19 cases had an impact on the healthcare sector. While the impact of COVID-19 has been mostly negative across several areas, the antibody therapy sector has gained significant attention as a result of a surge in demand for COVID-19 treatment development.
Several companies accelerated their R&D for the development of COVID diagnostic and treatment tools. The FDA and other international organizations granted emergency use authorizations (EUAs) for anti-SARS-CoV-2 mAb and other antibody therapy for the treatment of moderate to severe COVID-19 infection.
Such rapid product approvals for the treatment of COVID-19 have aided the growth of the antibody therapy industry during the pandemic. As a result, COVID-19 is expected to have a positive impact on the antibody drug market.
The Dynamic Landscape of COVID-19 Impact
Sector-Wide Interruption
The far-reaching impact of COVID-19 spared no sector, irrespective of its size, creating disruptions in the seamless operations that industries once enjoyed. The healthcare sector, a critical player in the battle against the pandemic, faced challenges and changes as it adapted to the evolving circumstances.
Antibodies Drug Market Therapy’s Resilience
While the prevailing narrative was one of adversity, the antibody therapy sector emerged as a beacon of resilience. The surge in demand for COVID-19 treatment development catapulted this sector into the limelight, showcasing its ability to navigate the challenges posed by the pandemic.
Accelerated R&D and Regulatory Responses
Swift Responses from Companies
Numerous companies swiftly pivoted, accelerating their Research and Development (R&D) efforts to contribute to the development of diagnostic and treatment tools for COVID-19. This collective effort aimed to address the urgent needs arising from the global health crisis.
Regulatory Green Lights
The FDA and other international organizations played a pivotal role by granting Emergency Use Authorizations (EUAs) for anti-SARS-CoV-2 monoclonal antibodies (mAb) and other antibody therapies. These authorizations specifically targeted the treatment of moderate to severe COVID-19 infections, paving the way for rapid advancements in therapeutic solutions.
Positive Impacts on Antibodies Drug Market Therapy
Rapid Approvals Propelling Growth
The swift approvals of products designed for treating COVID-19 played a crucial role in propelling the growth of the antibody therapy industry during the pandemic. This proactive response not only addressed immediate challenges but also positioned the industry for sustained positive impacts in the post-pandemic era.
Shaping the Future
The positive trajectory induced by the accelerated developments during the pandemic implies a lasting impact on the antibody drug market. COVID-19, despite its myriad challenges, has catalyzed innovations in healthcare, positioning the antibody therapy industry as a key player in addressing global health crises.
Market Scope And Categorization
The Increase In Human Antibodies Can Be Attributed To Increased Humira Demand And Sales
According to the source, human antibodies will dominate the global monoclonal antibody market in 2022. The introduction of Adalimumab biosimilar is anticipated to provide significant market opportunities during the forecast period. Increased pharmaceutical company investments in human mAb research, as well as increased demand for human antibodies, are propelling the market growth. Rising technological advancements to develop a molecular understanding of diseases, a rapidly aging population, and expanding applications of monoclonal antibodies are some of the factors driving the global monoclonal antibody market growth of human antibodies.
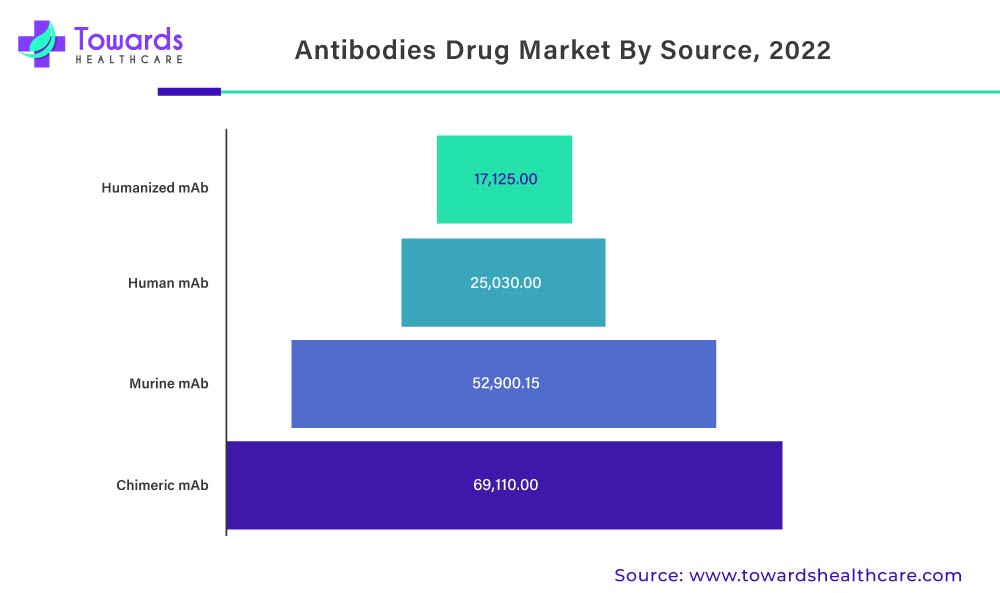
Demand For Monoclonal Antibodies Will Increase As Applications And Development Strategies Expand
Monoclonal antibodies are laboratory-made proteins that boost the immune system to fight against various harmful pathogens such as viruses.
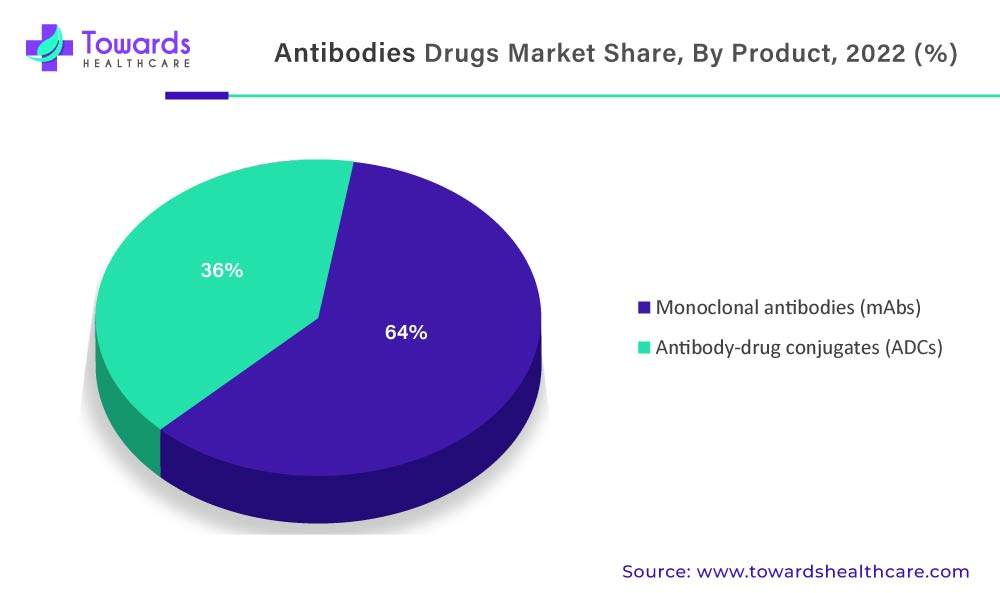
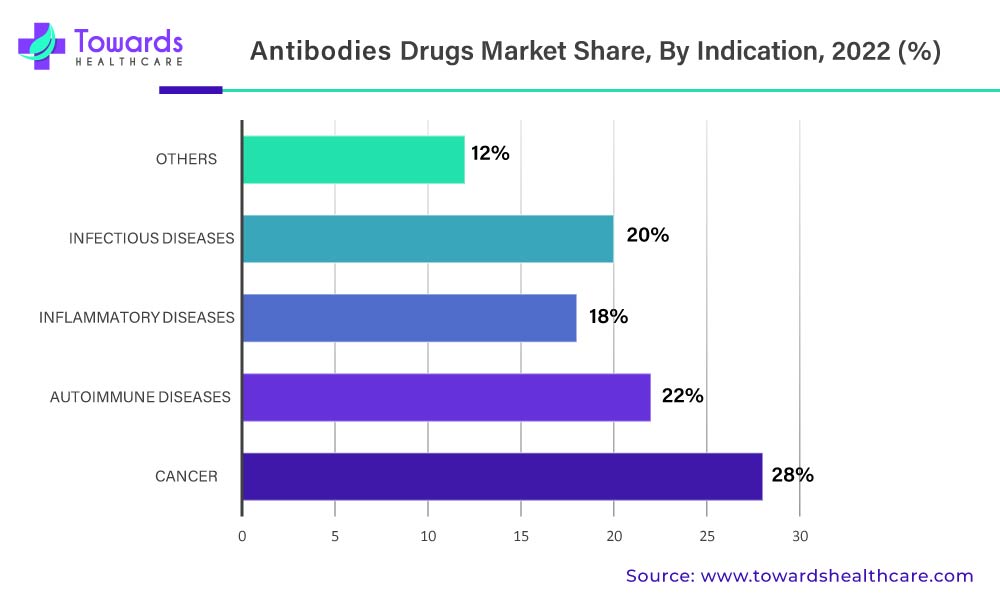
Due To The Increasing Prevalence And High Mortality Rate Of Cancer, There Is A Greater Need For Effective Antibodies Drug Market
Cancer will dominate the global antibodies drug market in 2022, according to indications. This increase is simply due to the increased prevalence of cancer and the rising demand for monoclonal antibodies in the treatment of various cancers such as lung cancer, breast cancer, colorectal cancer, and prostate cancer. The greater efficacy of mAbs in cancer treatment with few or no side effects is a major factor driving the high demand for monoclonal antibodies among cancer patients. Rising healthcare spending and increased awareness of mAbs along with their effectiveness in cancer treatment behold their dominance in the global market.
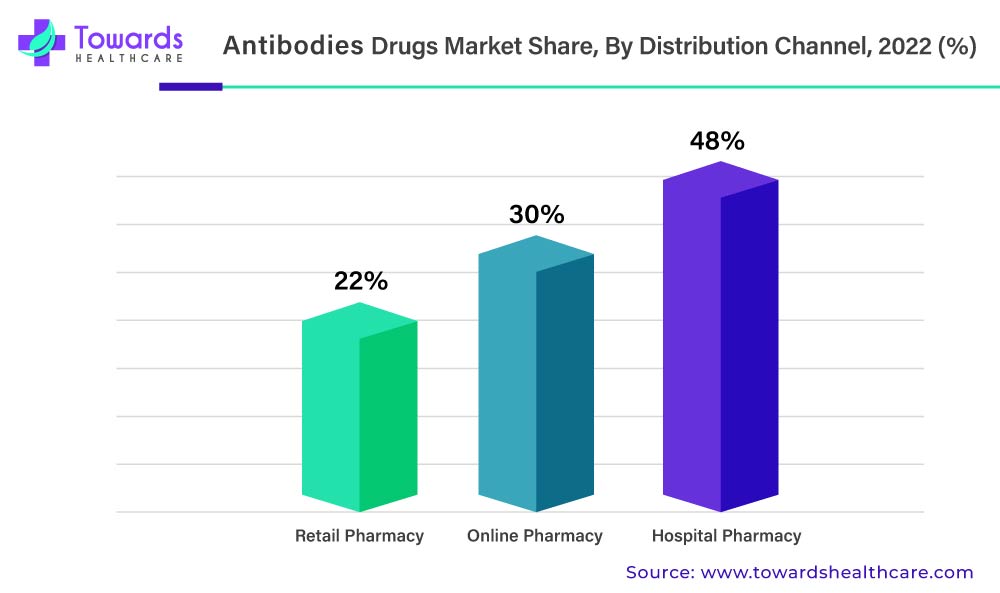
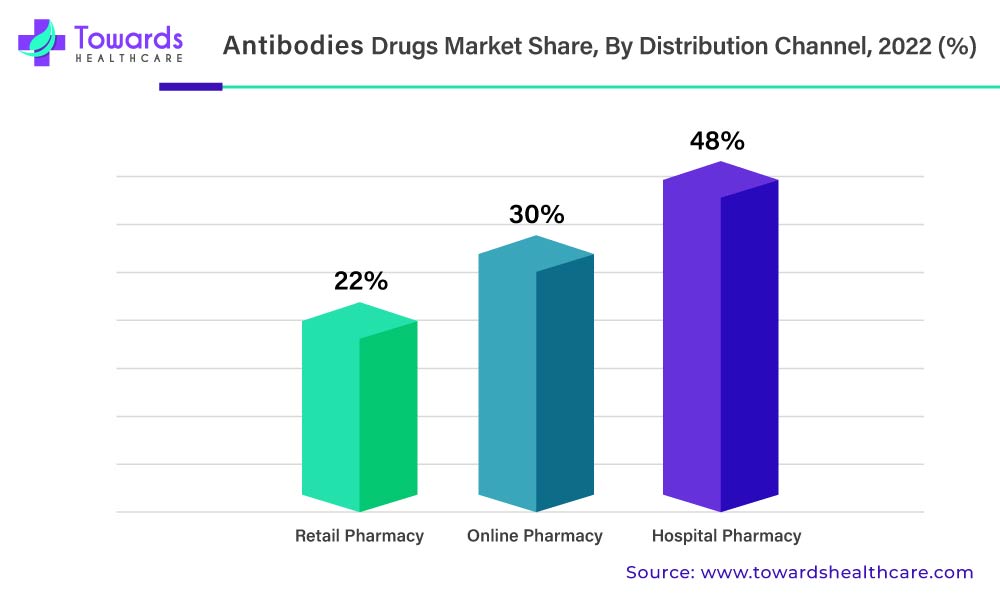
The Availability Of Advanced Treatment In Hospitals Will Drive Antibody Drug Demand
In 2022, the hospital pharmacies segment held the largest share of the antibodies drug market segment based on the distribution channel. This is due to an increase in hospital admissions due to the rising prevalence of chronic diseases such as cancer, autoimmune diseases, and rheumatoid arthritis among the population. The increased availability of advanced healthcare facilities has resulted in increased sales revenue for hospital pharmacies in the market. The wide variety of products and drugs available in hospital pharmacies makes it a convenient location for purchasing various drugs such as mAbs.
Rising Healthcare Expenditure And Cancer Incidence Will Boost The Size Of The North American Antibodies Drug Market
In 2022, North America will account for 36% of total revenue. The increased prevalence of chronic diseases in North America, the presence of advanced healthcare infrastructure, a rapidly aging population, high healthcare expenditure, and increased public awareness of mAbs have resulted in increased demand for monoclonal antibodies. According to the American Cancer Society, there will be over 1.8 million new cancer cases and approximately 606,520 deaths in the United States in 2020. By 2060, the US population is expected to have approximately 24% geriatric people aged 65 or older.
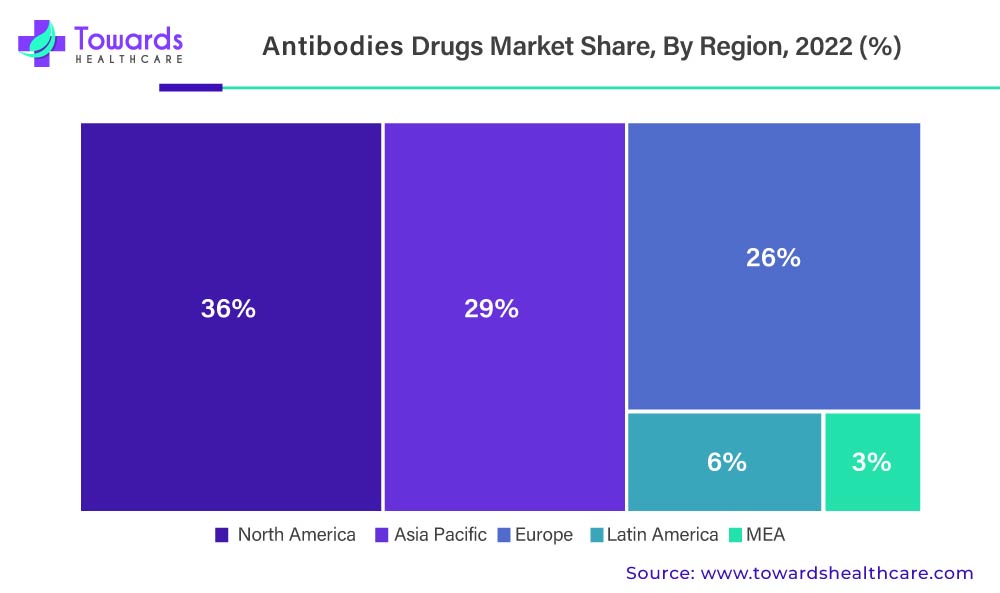
Why Does The United States Dominate The Global Antibodies Drug Market?
According to the Centers for Medicare & Medicaid Services (CMS) National Health Accounts report, healthcare spending in the United States increased by 4.6% in 2019. Rising cancer and autoimmune disease cases in the country will drive up the demand for antibodies even more.
According to the National Stem Cell Foundation (NSCF), autoimmune diseases are the third most common cause of chronic illness in the United States. The rising prevalence of diabetes and multiple sclerosis in the country will boost the demand for antibodies.
Therapeutic Antibodies Are Currently Being Tested In Clinical Trials
Companies are currently funding clinical trials for over 570 mAbs. Approximately 90% of these are early-stage studies designed to assess patient populations’ safety (Phase I) or safety and preliminary efficacy (Phase I/II or Phase II). The majority of mAbs in Phase I (70%) are for cancer treatment, and the proportions of mAbs in Phase II and late-stage clinical studies (pivotal Phase II, Phase II/III, or Phase III) are similar.
Recent Development
- Amgen and AstraZeneca have announced the approval and availability of TEZSPIRE (tezepelumab-ekko) for the treatment of serious asthma in January 2022. Such FDA drug approval will allow the company to expand its product portfolio in the industry and generate significant revenue.
- Regeneron announced the Emergency Use Authorization (EUA) by the US FDA for its antibody cocktail casirivimab and imdevimab developed for COVID-19 treatment in November 2020. This approval has boosted the company’s revenue generation and helped it gain a significant market share.
- In December 2021, Roche announced the launch of the AVENIO Edge System, a key component of Roche’s sequencing technology advancement strategy. Built on best-in-class foundational capabilities to deliver a fully-automated, combined sequencing solution. The AVENIO Edge System is a pre-analytical platform for sequencing library preparation, target enrichment, and quantification steps that deliver integrated, end-to-end regulation with consistent, high-quality results.
- Roche announced the launch of the cobas® 5800 System, a novel molecular laboratory tool, in countries that accept the CE mark in November 2021. Testing is one of the first lines of defense in ensuring a patient’s general well-being and is critical in guiding their treatment as quickly as possible.
- Roche announced the CE-IVD introduction of its automated digital pathology algorithms, uPath HER2 (4B5) image analysis, and uPath Dual ISH image analysis for breast cancer in January 2021 to assist in determining the best treatment strategy for each patient. Artificial intelligence is used in image analysis algorithms to assist pathologists in making faster, more precise patient diagnoses of breast cancer. A mutation in the HER2 gene, which occurs in up to 20% of the 2.1 million cases of breast cancer diagnosed worldwide each year, is responsible for intrusive development in some patients.
- Amgen launched Amgevita, an adalimumab biosimilar, in Europe in October 2018.
- CARsgen Therapeutics received IND (Investigational New Drug) approval for its AB011 humanized mAb in China in December 2019 for the treatment of pancreatic and gastric adenocarcinoma.
The global antibodies drug market monumental rise from USD 200.18 billion in 2022 to the anticipated surpassing of USD 605.8 billion by 2032 is a testament to the evolving landscape of healthcare. Fueled by the surge in monoclonal antibodies production, the market is not only meeting current demands but also positioning itself as a crucial player in future healthcare solutions. As the industry continues to evolve, stakeholders must adapt to these dynamic changes, ensuring that the antibodies drug market remains at the forefront of innovative and impactful healthcare interventions.