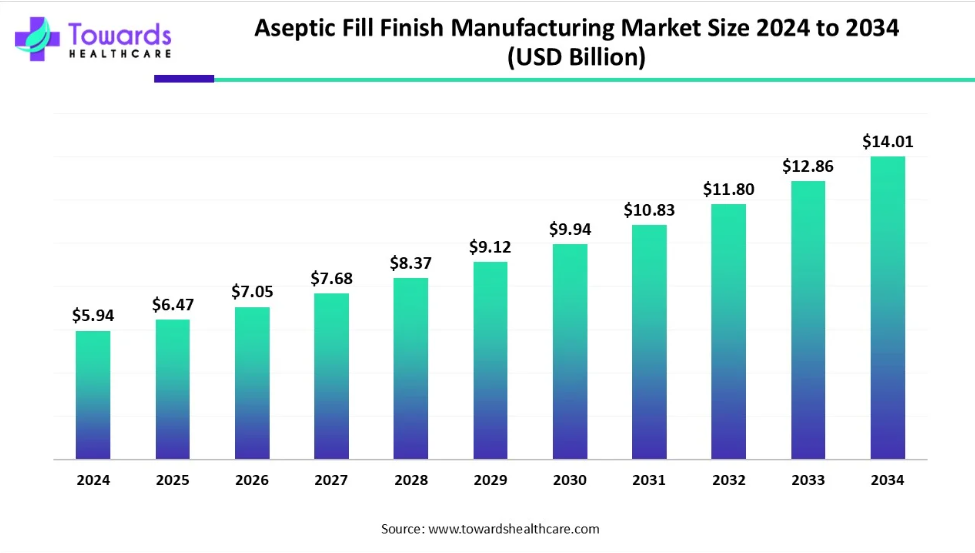
The global aseptic fill finish manufacturingmarket is projected to witness substantial growth, reaching USD 14.01 billion by 2034, from USD 5.94 billion in 2024 and USD 6.47 billion in 2025. This reflects a CAGR of 8.94% between 2025 and 2034, driven by the rising demand for sterile pharmaceutical formulations, biologics, and vaccines.
Get All the Details in Our Solutions – Download Brochure: https://www.towardshealthcare.com/download-brochure/5549
📊 Market Snapshot (2024–2034)
🔑 Key Takeaways
- Region-wise:
- North America leads the market in 2024.
- Asia-Pacific is projected to be the fastest-growing region.
- Molecule Type:
- Biologics segment dominated in 2024.
- Packaging Container Type:
- Ampoules held the largest share.
- Vials are projected to grow the fastest.
- Drug Products:
- Vaccines led the market in 2024.
- Therapeutic Area:
- Autoimmune disorders held a significant share.
- Oncological disorders to grow at the highest CAGR.
- Scale of Operation:
- Preclinical/clinical segment dominated.
- Commercial segment is expected to grow rapidly.
🧪 What is Aseptic Fill Finish Manufacturing?
Aseptic fill finish is a critical sterile process in pharmaceutical production where a drug product is filled into containers—vials, ampoules, or syringes—without compromising sterility. This process is pivotal for:
- Biologics
- Vaccines
- Monoclonal antibodies
- Antibiotics and antibody-drug conjugates
It ensures regulatory compliance, patient safety, and product efficacy.
🚀 Market Drivers
✔️ Increasing Collaborations
Biotech and pharma startups are increasingly partnering with CMOs and CDMOs to leverage their aseptic capabilities, reducing cost and time-to-market.
⚠️ Market Restraint
❌ Sterility Challenges
Maintaining a contamination-free environment is complex and costly. A single breach can lead to batch rejection and heavy financial loss.
💡 Market Opportunity
🔁 Rise of Single-Use Technology
- Disposable systems eliminate the need for post-process sterilization.
- Reduce contamination risk and operational cost.
- Offer flexibility, regulatory compliance, and environmental benefits.
🤖 AI Integration in Aseptic Fill Finish
Artificial intelligence is revolutionizing the sterile fill-finish space:
- Automation of processes reduces human error and contamination.
- Predictive analytics help preempt failures and optimize operations.
- 3D vision systems and AI-enabled robotics enhance visual inspection, filling accuracy, and reproducibility.
- Smart maintenance improves equipment longevity and reduces downtime.
🔄 Market Trends
| Date | Event |
|---|---|
| Feb 2025 | Recipharm unveiled a modular sterile filling system for pilot to clinical-scale batches. |
| Nov 2024 | Adragos Pharma acquired Baccinex to expand fill-finish capabilities. |
| Sep 2024 | Symbiosis Pharma acquired a 43,500 sq. ft. site in Stirling, Scotland, for expansion. |
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
📈 Segmental Insights
🔬 Molecule Type: Biologics
Biologics like vaccines, recombinant proteins, and gene therapies dominate due to rising demand for targeted and personalized treatment.
💉 Packaging Type:
- Ampoules are preferred for their tamper-proof, hermetically sealed structure.
- Vials are gaining popularity due to their flexibility and reusability.
💊 Drug Products: Vaccines
The global focus on infectious disease prevention and immunization programs is driving this segment.
🧬 Therapeutic Areas:
- Autoimmune Disorders: Demand for biologics and injectable treatments like insulin drives growth.
- Oncological Disorders: Increasing cancer cases globally fuel the need for sterile cancer therapeutics.
🧪 Scale of Operation
| Segment | Insights |
|---|---|
| Preclinical | Driven by growing clinical trials and R&D investments. |
| Commercial | Rising demand post-approval pushes the need for large-scale, compliant manufacturing. |
🌎 Regional Overview
🇺🇸 North America
- Strong government funding (e.g., BARDA, DoD) and FDA approvals boost market.
- Demand for personalized therapies and robust CDMO presence support growth.
🇨🇦 Canada
- Over $2.3 billion invested in 41 biomanufacturing projects (2020–2023).
- Strategic infrastructure and funding via SIF and NRC IRAP promote fill-finish capabilities.
🌏 Asia-Pacific
- Low-cost manufacturing infrastructure, FDI encouragement, and skilled labor fuel market expansion.
- Attractive for global pharma companies looking to expand operations.
To invest in our premium strategic solution and customized market report options, click here: https://www.towardshealthcare.com/price/5549