Bioprocess Bags Market Growth, Trends and Regional Insights
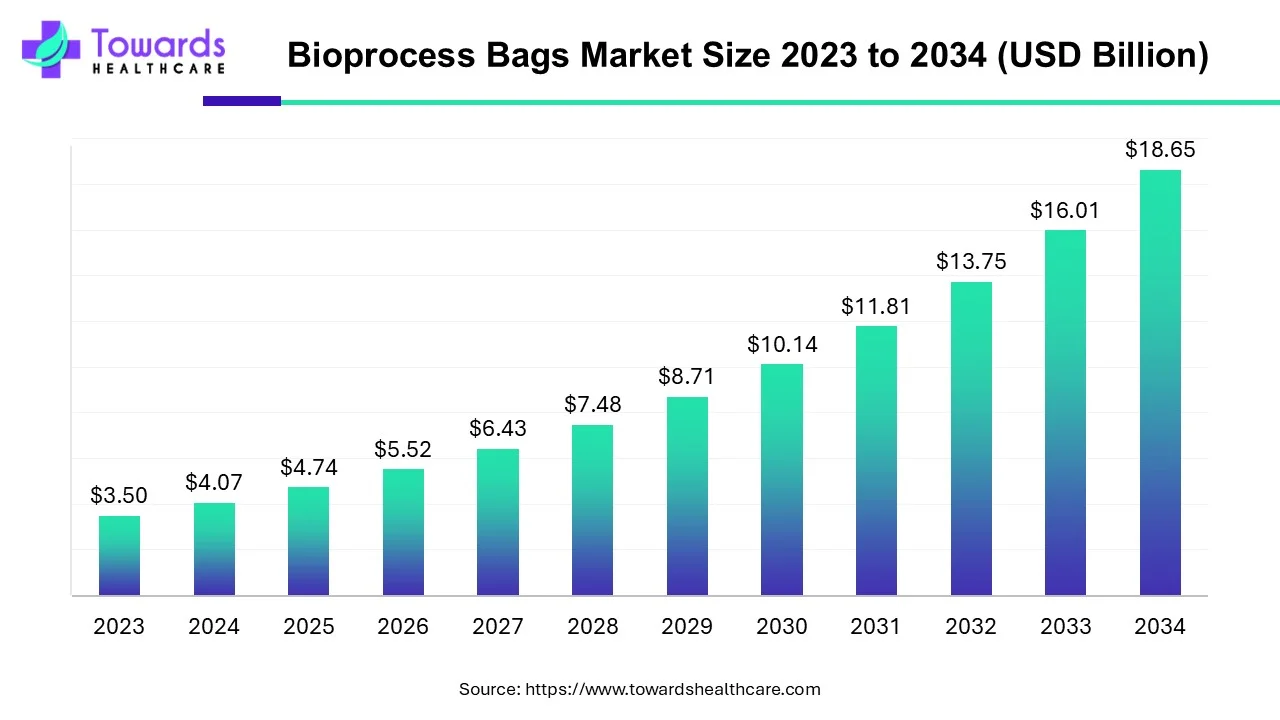
The global bioprocess bags market was valued at USD 4.07 billion in 2024 and is projected to grow to USD 4.74 billion in 2025. By 2034, the market is expected to reach approximately USD 18.65 billion, growing at a compound annual growth rate (CAGR) of 16.44% from 2025 to 2034. This significant growth is driven by the expanding biotechnology industry, the increasing demand for biologics, and ongoing technological advancements.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5398
Bioprocess Bags Market: Key Drivers and Applications
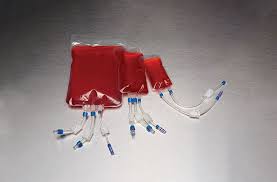
Bioprocess bags are single-use, flexible, and customizable solutions used in bioprocess environments to safely handle liquids. Made from multilayer films, they offer a variety of properties, including biocompatibility, chemical resistance, strength, and effective barriers. These bags are used across the biopharmaceutical industry, from small-scale research to large-scale production.
They serve various functions, such as collecting, feeding, harvesting, storing, and transporting media, buffers, cell cultures, and products related to cell and gene therapies. They are also crucial in cryopreservation, providing storage at extremely low temperatures using liquid nitrogen.
The growth of the biotechnology sector, alongside the increasing focus on the production of biologics, is a key factor in the market’s expansion. Additionally, rising instances of chronic and acute diseases are fueling research and development, which is driving demand for bioprocess bags. As the pharmaceutical industry seeks more efficient and sterile storage solutions throughout the drug manufacturing process, the demand for bioprocess bags continues to rise. The integration of advanced manufacturing technologies, including automation, further enhances production capabilities and market growth.
Bioprocess Bags Market Trends
- Growth in Biopharmaceuticals and Biologics
The demand for biologics, including monoclonal antibodies, vaccines, and gene therapies, is a major driver of the bioprocess bags market. As the biopharmaceutical industry expands, especially with the increased focus on cell and gene therapies, the need for bioprocess bags for safe handling and storage of these products grows. - Single-Use Technology Adoption
The trend towards single-use technologies in the biopharmaceutical sector is gaining momentum. Bioprocess bags offer a cost-effective and convenient solution for various applications, such as media preparation, cell culture, and buffer solutions, reducing the risk of cross-contamination and the need for cleaning. - Customization and Flexibility
The demand for customizable bioprocess bags is on the rise. Manufacturers are developing bioprocess bags in a variety of sizes, shapes, and materials to meet the specific needs of different bioprocesses, from research and development to full-scale production. This trend supports greater efficiency and versatility in biomanufacturing. - Technological Advancements
Advances in bioprocessing technologies, including automation and enhanced film materials, are improving the functionality and performance of bioprocess bags. For example, some bioprocess bags now feature integrated sensors and monitoring systems to track parameters such as temperature, pressure, and pH, providing greater control during the biomanufacturing process. - Cryopreservation and Cold Chain Expansion
The increasing use of bioprocess bags for cryopreservation, especially in the storage of cell lines, vaccines, and other biological products, is a key market trend. The growth of cold chain logistics and storage solutions further supports the need for bioprocess bags capable of handling liquid nitrogen temperatures for long-term storage. - Regional Market Growth
The North American and European markets continue to dominate, but the Asia-Pacific region is experiencing rapid growth. Countries like China and India are becoming major hubs for biopharmaceutical manufacturing, driving demand for bioprocess bags in the region. - Regulatory Compliance and Quality Standards
Increasing emphasis on regulatory compliance and quality standards in the production of biologics and pharmaceuticals is pushing the demand for bioprocess bags that meet strict safety and performance criteria. This includes ensuring that the materials used in these bags do not interact with biological products, maintaining sterility throughout the manufacturing process.