Biosimilars Market Growth and Outlook
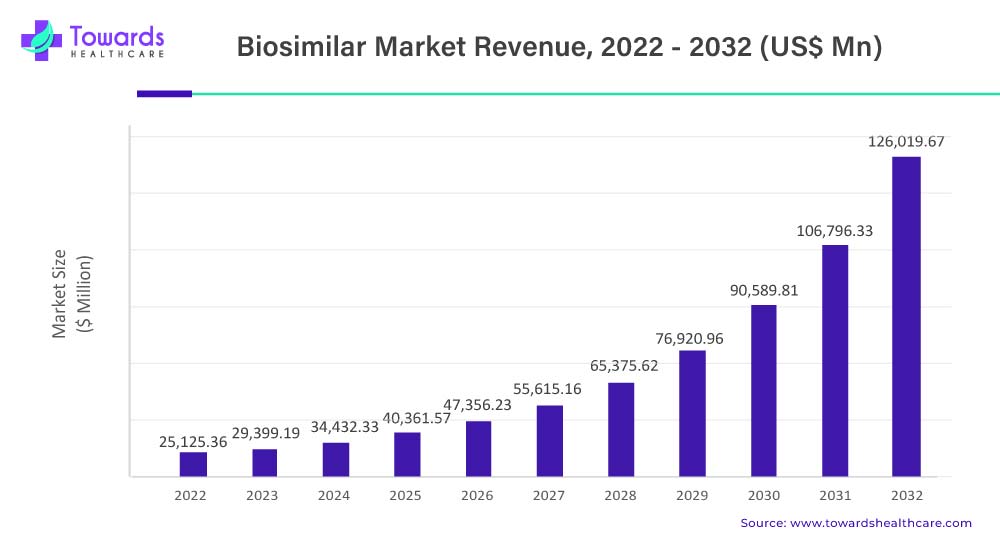
The global biosimilars market, valued at USD 25.1 billion in 2022, is projected to grow at a 17.6% CAGR, reaching USD 126 billion by 2032. This growth is driven by the rising cancer rates and the demand for cost-effective treatments.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5035
The Growing Impact of Biosimilars in Healthcare and Cancer Treatment
In 2021, 91% of the 6.4 billion prescriptions written in the United States were for generic or biosimilar medications, with biosimilars alone saving the healthcare system $7 billion. Biosimilars are biological products that closely resemble FDA-approved reference products and have no significant clinical differences. Also known as follow-on biologics or biogenerics, biosimilars are made from living cells, which may cause slight differences in manufacturing, but they demonstrate similar safety, efficacy, and quality as their reference drugs through extensive clinical trials.
As of September 2022, 39 biosimilars have been approved, and 22 are on the market. These drugs offer substantial cost savings, typically 10-40% less than their reference biologics, making them a cost-effective treatment option for patients and healthcare providers. The biosimilar market has seen rapid growth, fueled by the expiration of patents for several biologic drugs and a rising demand for more affordable alternatives.
Developing biosimilars is a complex process. They undergo rigorous regulatory procedures to demonstrate their similarity to reference products, including head-to-head clinical trials. Biosimilars are used to treat a range of chronic conditions, including rheumatoid arthritis, anemia, inflammatory bowel disease, psoriasis, and cancer. They offer an opportunity for broader patient access, improving adherence and health outcomes.
In fact, FDA-approved biosimilars could save the healthcare system up to $250 billion in their first decade on the market. These savings stem from both the direct cost reduction of biosimilars and the increased competition that drives down prices for biologics.
Biosimilars are particularly promising in cancer treatment. Biologic drugs, often used in oncology, are expensive, limiting access to life-saving treatments for many patients, especially in developing countries. Biosimilars offer a more affordable alternative without sacrificing safety or effectiveness. Several biosimilars have been approved for cancer treatments, including those for breast cancer, colorectal cancer, and non-Hodgkin’s lymphoma. Biosimilars of granulocyte colony-stimulating factor (G-CSF) are also used to treat neutropenia, a common chemotherapy side effect.
In 2022, oncology applications accounted for the largest share of the biosimilars market, at around 24.29%, and are expected to continue growing rapidly in the coming years.
Biosimilars Market Top Companies
- Novartis
- Synthon Pharmaceuticals, Inc.
- TevaPharmaceutical Industries Ltd.
- LG Life Sciences
- Celltrion
- Biocon
- Hospira
- Merck Serono
- Biogen idec, Inc.
- Genentech
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/biosimilars-market
Access exclusive insight now @ https://www.towardshealthcare.com/price/5035
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare