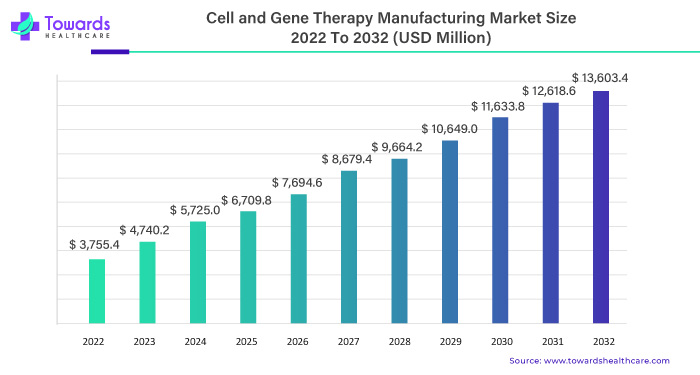
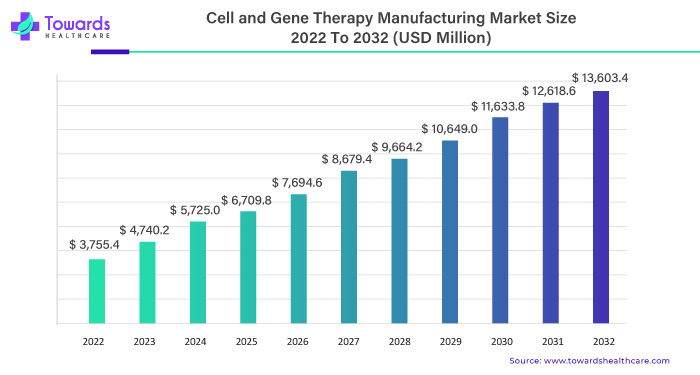
In the dynamic landscape of healthcare, the cell and gene therapy manufacturing market emerge as a pivotal force, steering an extraordinary surge. Starting at an estimated USD 3,755.4 million in 2022, this market is set to ascend to a staggering USD 13,603.4 million by 2032. The journey is characterized by a robust Compound Annual Growth Rate (CAGR) of 16.25% between 2023 and 2032.
Evolutionary Dynamics: Unleashing the Potential of Cell and Gene Therapy Manufacturing Market
Genesis and Transformations
The narrative of cell and gene therapy manufacturing market unfolds as a saga of evolution and transformation. From its inception to the contemporary landscape, the sector has witnessed significant advancements, reshaping the contours of medical innovation.
Market Size Revelation: Navigating Financial Horizons
Commencing at USD 3,755.4 million in 2022, the cell and gene therapy manufacturing market is poised to unveil an unprecedented financial horizon, reaching an estimated USD 13,603.4 million by 2032. This monumental leap reflects the sector’s robust growth trajectory and its integral role in shaping the future of healthcare.
CAGR Odyssey: Charting a Course of Sustained Growth
At the heart of this surge lies a compelling Compound Annual Growth Rate (CAGR) of 16.25%. Between 2023 and 2032, the market is slated to experience an exponential expansion, marking an era of sustained growth and groundbreaking innovations.
Considering its potential to treat cancer, cell and gene therapy has sparked a lot of interest. With a complicated and expanding sector, a Contract Development and Manufacturing Company (CDMO) may assist customers in smoothing commercial cell and gene therapy manufacturing market needs.
In August 2022, Cytiva, a subsidiary of Danaher Corporation, and Forecyte Bio, a provider of CDMO service designed for cell gene therapy, collaborated to accelerate the development and manufacturing of cell and gene therapies in China and the U.S. Forecyte Bio will use Cytiva’s Flex Factory platform to launch its contract development and manufacturing organization (CDMO) business.
Ill effects of COVID-19 on the Cell and Gene Therapy Manufacturing Market
Due to the COVID-19 pandemic, players in the worldwide cell therapy manufacturing industry are experiencing significant hurdles on several fronts. The key obstacles are raw material availability owing to transportation facility inconsistencies. Furthermore, because of the increased prevalence of COVID -19, distributors are experiencing unpredictable demand for products from merchants.
However, there has been a modest positive influence on the market since cell therapies for the treatment of COVID-19 are being researched.
According to a Springer Nature article, mesenchymal cells (MSCs) are now being explored as one of the therapeutic strategies for COVID-19 illness. Researchers utilize immunosuppressive medications (Tocilizumab) to treat COVID-19 symptoms in patients; these cells can greatly improve critical circumstances due to their mode of action.
Influencers Fueling the Current Market Scenario
Key Players are involved in developing and launching various types of technologies for cell therapy manufacturing, which is expected to provide immense growth opportunities for industry players in the cell therapy manufacturing market. Manufacturers of cell therapy product candidates are engaged in receiving regulatory approvals to enhance their cell therapy manufacturing process, which in turn is expected to drive the cell therapy manufacturing market growth
Hurdles Faced During Market Growth
One of the most significant issues is the high cost of cell treatments, which has financial consequences for patients, payers, and providers. As a result, firms must lower their pricing in order to increase the acceptance of these medicines, which is projected to hamper market growth throughout the forecast period.
Open Doors for the Cell and Gene Therapy Manufacturing Market
Soon, the cell therapy manufacturing market is likely to rise due to increased investment by major companies in the development of improved cell treatments.
For instance, in January 2022, Cellino Biotech, Inc., an autonomous cell therapy manufacturing company, announced the completion of a Series A financing of US$ 80 million, led by the impact investment arm of Bayer AG —Leaps by Bayer— 8VC, and Humboldt Fund. New investors in the round include Felicis Ventures and others, joining existing investors The Engine and Khosla Ventures. The company has raised a total of US$ 96 million in gross proceeds from private financings.
Market Scope and Categorization of the Cell and Gene Therapy Manufacturing Market
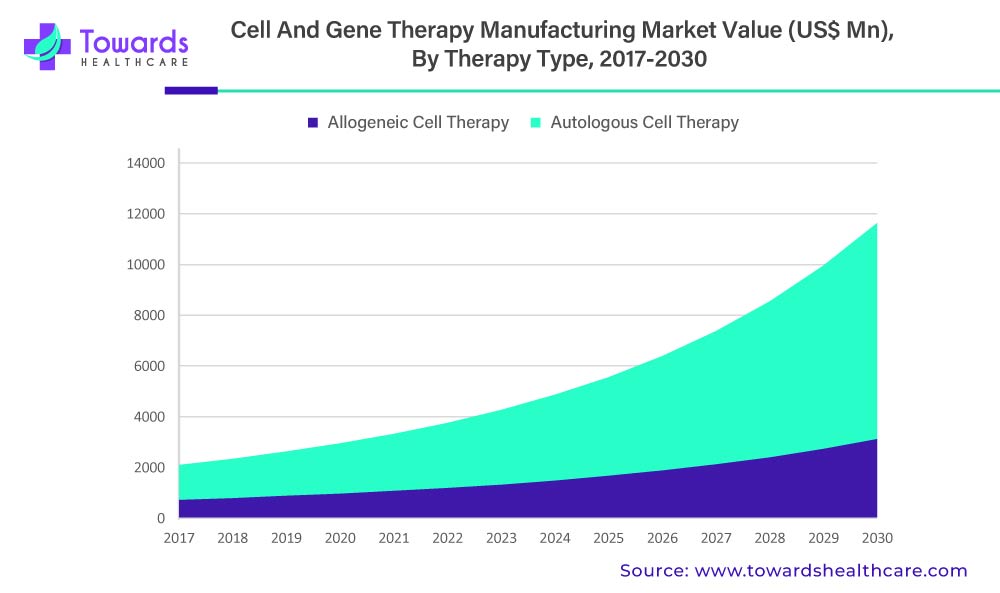
The allogeneic cell therapy segment is estimated to be valued at US$ 1,198.2 Mn in 2022 and is expected to reach US$ 3,129.6 Mn by 2030 at a CAGR of 12.8%.
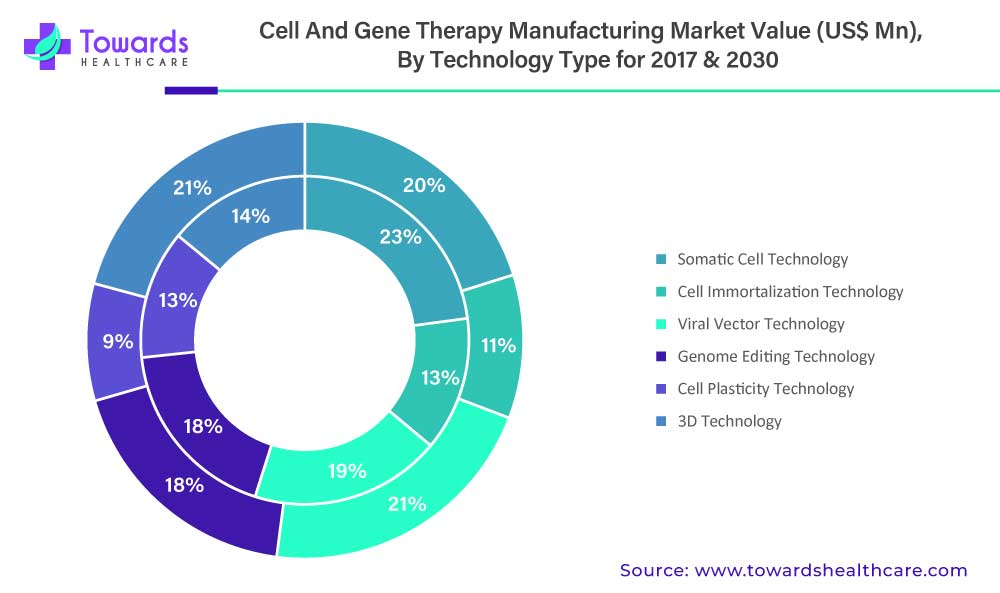
The somatic cell technology segment is estimated to be valued at US$ 823.2 Mn in 2022 and is expected to reach US$ 2,345.6 Mn by 2030 at a CAGR of 14.0%.
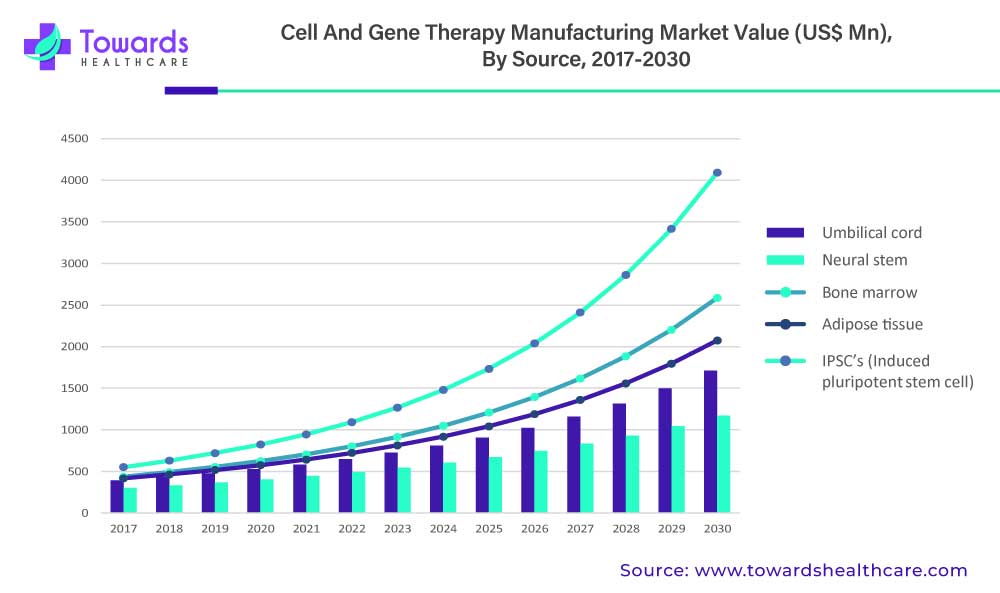
IPSC’s (Induced pluripotent stem cell) segment is estimated to be valued at US$ 1,092.5 Mn in 2022 and is expected to reach US$ 4,092.3 Mn by 2030 at a CAGR of 17.9%.
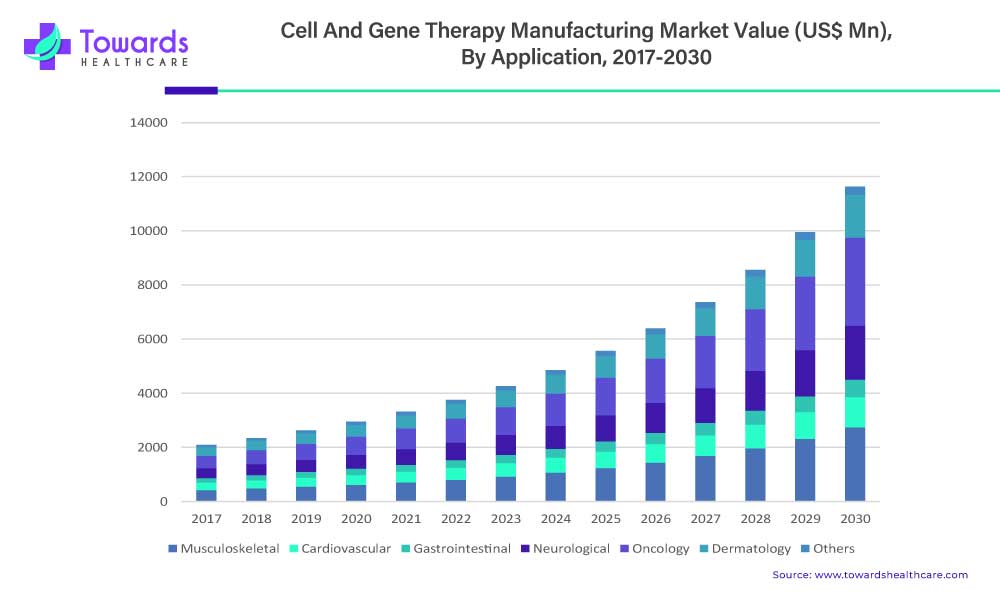
 The musculoskeletal segment is estimated to be valued at US$ 807.3 Mn in 2022 and is expected to reach US$ 2,740.2 Mn by 2030 at a CAGR of 16.5%
The musculoskeletal segment is estimated to be valued at US$ 807.3 Mn in 2022 and is expected to reach US$ 2,740.2 Mn by 2030 at a CAGR of 16.5%
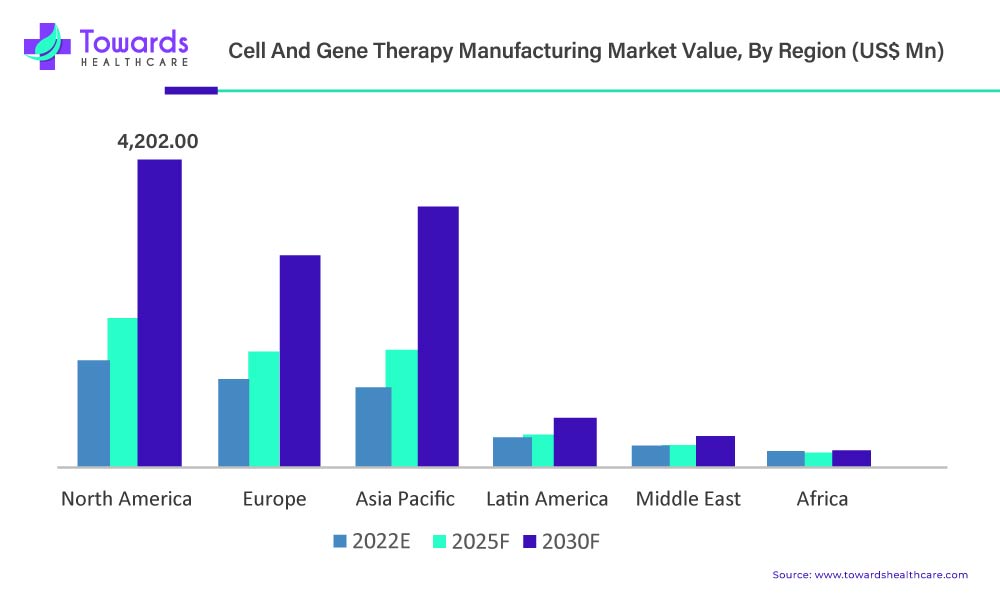
The North American cell therapy manufacturing market is estimated to be valued at US$ 1,326.8 Mn in 2022 and is projected to increase to US$ 4,202.0 Mn by 2030, witnessing a CAGR of 15.5% between 2022 and 2030.
Global Cell Therapy Manufacturing Market Shere, By Region, 2022 and 2030
| Region | Global Cell Therapy Manufacturing Market Shere, By Region, 2022 | Global Cell Therapy Manufacturing Market Shere, By Region, 2030 |
| North America | 35.30% | 36.10% |
| Lattin America | 6.10% | 4.90% |
| Europe | 28.10% | 24.80% |
| Asia Pacific | 25.80% | 30.90% |
| Middle East | 3.40% | 2.50% |
| Africa | 1.20% | 0.80% |
Major key players’ strategic initiatives for shaping business growth
| Key Players | Strategies |
| Partnership | On June 15, 2022, WuXi Advanced Therapies, Contract Testing, Development, and Manufacturing Organization (CTDMO) business unit of WuXi AppTec. and Wugen Inc., a clinical-stage biotechnology company, announced a partnership to manufacture Wugen’s WU-NK-101, novel immunotherapy that harnesses the power of memory natural killer (NK) cells to treat cancer. WuXi ATU will provide manufacturing and testing services for WU-NK-101 to enable the delivery of this innovative cell therapy product to cancer patients. |
| Announcement | In May, WuXi Advanced Therapies, a contract development and manufacturing organization (CDMO) of WuXi AppTec., announced that it had expanded its service capabilities to offer a fully integrated, closed-process CAR-T cell therapy platform. This new platform will shorten the timeframe for developing, manufacturing, and delivering cell and gene therapies while providing better predictability. |
| Rebranding | In July 2019, BioTime, Inc. announced that it had introduced a new corporate brand, including a change of its corporate name to Lineage Cell Therapeutics, Inc. effective from August 12, 2019. The company also relocated its corporate headquarters to Carlsbad, California, effective August 12, 2019. |
| Collaboration | In June 2018, BioTime Inc. company entered into an agreement with Athersys, Inc., a biotechnology company, for expanding its collaboration in additional therapeutic areas |
| Acquisition | In October 2019, Merck KGaA announced that it had acquired FloDesign Sonics of Wilbraham, Massachusetts, a developer of a unique acoustic cell processing platform, for the industrialization of cell and gene therapy manufacturing |
| Agreement | On June 21, 2021, Takara Bio Inc. announced that it had entered into a license and supply agreement with BioNTech, a Germany-based biotechnology company. Takara Bio grants BioNTech a commercial license to use applicable patents relating to RetroNectin. Under this agreement, Takara Bio provides BioNTech with reliable supplies of RetroNectin, which can be used to manufacture cell and gene therapy products. |
Driving Forces: Factors Propelling Growth
Technological Innovations
The rapid evolution of technology serves as a catalyst for the unprecedented growth in cell and gene therapy manufacturing market. Cutting-edge innovations propel the sector forward, unlocking new dimensions in medical science.
Rising Therapeutic Efficacy
As the efficacy of cell and gene therapies continues to rise, there is a paradigm shift in the healthcare landscape. The transformative impact of these therapies not only enhances patient outcomes but also contributes significantly to the market’s upward trajectory.
Regulatory Support
A conducive regulatory environment plays a pivotal role in fostering growth within the cell and gene therapy manufacturing market. Supportive regulatory frameworks empower researchers and businesses, fostering an environment conducive to breakthroughs and advancements.
Emerging Trends Shaping Cell and Gene Therapy Manufacturing Market
Advanced Manufacturing Technologies
The cell and gene therapy manufacturing market is witnessing a surge in advanced manufacturing technologies. Automation, robotics, and machine learning are revolutionizing production processes, ensuring scalability, efficiency, and precision in the manufacturing of these groundbreaking therapies.
Personalized Medicine Revolution
Cell and gene therapies are at the forefront of personalized medicine, tailoring treatments to individual patients based on their genetic makeup. This paradigm shift from one-size-fits-all to precision medicine marks a revolutionary advancement in healthcare, offering targeted and more effective therapeutic interventions.
Increased Funding and Investment
The growing recognition of the potential of cell and gene therapies has attracted substantial funding and investment. Governments, private investors, and pharmaceutical companies are pouring resources into research and development, manufacturing infrastructure, and clinical trials, fostering an environment conducive to innovation.
Addressing Challenges in Cell and Gene Therapy Manufacturing Market
Scalability and Commercialization
As the demand for cell and gene therapies rises, ensuring scalability and effective commercialization becomes a critical challenge. Innovations in scalable manufacturing processes are essential to meet the increasing global demand for these transformative therapies.
Regulatory Frameworks and Standardization
The regulatory landscape for cell and gene therapies is evolving rapidly. Establishing clear and standardized regulatory frameworks is crucial for ensuring the safety, efficacy, and quality of these therapies. Collaboration between regulatory bodies and industry stakeholders is essential to navigate this complex terrain.
Infrastructure Development
The need for specialized infrastructure for cell and gene therapy manufacturing market is paramount. Investing in state-of-the-art facilities equipped with the latest technologies is essential to meet the rigorous standards and requirements of manufacturing these sophisticated therapies.
Collaborative Initiatives and Global Partnerships
Industry Collaboration
Collaboration within the industry is a driving force behind the advancements in cell and gene therapy manufacturing market. Companies are forming strategic partnerships to share expertise, resources, and best practices, accelerating the development and commercialization of these therapies.
Academic and Research Collaborations
Academic institutions and research organizations play a pivotal role in advancing the science and technology behind cell and gene therapies. Collaborative initiatives between academia and industry facilitate knowledge exchange, propelling the sector forward.
International Cooperation
The global nature of healthcare challenges calls for international cooperation. Collaborative efforts on a global scale enable the sharing of research findings, regulatory insights, and manufacturing capabilities, fostering a collective approach to advancing cell and gene therapy manufacturing market.
Future Outlook: A Landscape of Infinite Possibilities
The future of cell and gene therapy manufacturing market holds tremendous promise. As technology continues to evolve, and regulatory frameworks mature, the sector is poised for unprecedented growth. The convergence of scientific innovation, strategic collaboration, and global initiatives positions cell and gene therapy manufacturing market as a cornerstone in the future of healthcare. The journey ahead promises not only economic prosperity but also, more importantly, a profound impact on improving patient outcomes and transforming the treatment landscape for numerous diseases.
Overcoming Manufacturing Complexities
Viral Vector Production
Viral vectors are crucial components in many gene therapies. Enhancements in viral vector production processes are pivotal for the efficient and cost-effective manufacturing of gene therapies, addressing challenges related to scalability and production yield.
Supply Chain Optimization
Optimizing the supply chain for cell and gene therapies is a key consideration. From sourcing raw materials to distribution, ensuring a streamlined and reliable supply chain is essential for meeting the increasing demand for these specialized therapies.
Quality Control and Assurance
Maintaining stringent quality control measures is paramount in cell and gene therapy manufacturing market. Advanced analytics, monitoring systems, and quality assurance protocols are continuously evolving to meet the high standards required for these advanced therapies.
Patient Access and Affordability
Market Access Strategies
Ensuring broad patient access to cell and gene therapies requires strategic market access planning. Collaborations with payers, healthcare providers, and patient advocacy groups are essential to navigate reimbursement processes and make these therapies accessible to a wider patient population.
Affordability Considerations
The high costs associated with cell and gene therapies pose challenges to widespread adoption. Ongoing efforts to explore innovative pricing models, reimbursement strategies, and cost-sharing initiatives aim to address concerns related to the affordability of these groundbreaking treatments.
Regulatory Landscape and Global Harmonization
Regulatory Evolution
The regulatory environment for cell and gene therapies is dynamic and continually evolving. Regulatory bodies worldwide are adapting to the unique characteristics of these therapies, providing guidance and frameworks to facilitate their development, approval, and commercialization.
Global Harmonization Efforts
Harmonizing regulatory standards on a global scale is a critical focus. International collaboration among regulatory agencies aims to establish common guidelines, streamline approval processes, and ensure consistency in regulatory requirements for cell and gene therapies.
Ethical and Societal Implications
Ethical Considerations
The ethical implications of manipulating genes and cells raise important considerations. Ethical frameworks and ongoing dialogue among researchers, clinicians, ethicists, and the broader public are essential to navigate the ethical landscape surrounding cell and gene therapies.
Societal Impact
The broader societal impact of widespread adoption of cell and gene therapies is multifaceted. Addressing issues such as equitable access, societal acceptance, and ethical concerns is crucial to ensure that the integration of these therapies aligns with societal values and expectations.
Research Frontiers and Innovation
Emerging Therapeutic Applications
Research in cell and gene therapy extends beyond current applications. Ongoing exploration of novel therapeutic targets and innovative applications, including RNA-based therapies and gene editing technologies, continues to push the boundaries of what is achievable in healthcare.
Next-Generation Technologies
Advancements in next-generation technologies, such as CRISPR-based gene editing and RNA-based therapies, hold immense promise. These technologies are at the forefront of research, offering the potential for more precise, targeted, and customizable therapeutic interventions.
In conclusion, the realm of cell and gene therapy manufacturing market is a dynamic and multifaceted landscape. As the industry navigates complexities, embraces ethical considerations, and pushes the boundaries of innovation, the future holds unprecedented potential for transformative healthcare solutions that have the power to revolutionize patient care on a global scale.
Continuing the exploration of cell and gene therapy manufacturing market, ongoing research endeavors focus on enhancing the efficiency of delivery mechanisms, reducing production costs, and optimizing therapeutic outcomes. Innovations in CRISPR technology promise to revolutionize gene editing precision, opening new possibilities for targeted and personalized treatments. Additionally, collaborations between academia, industry, and healthcare providers are fostering interdisciplinary research, driving advancements in regenerative medicine and stem cell therapies. As the landscape evolves, a commitment to ethical considerations, patient-centricity, and global collaboration will be pivotal in ensuring that cell and gene therapies continue to shape the future of medicine while addressing the diverse challenges and opportunities that lie ahead.
Future Horizons: Anticipating Expansion
Global Outreach
The cell and gene therapy manufacturing market transcends geographical constraints, with a global outreach that connects researchers, manufacturers, and healthcare providers worldwide.
Diversification of Therapeutic Applications
As research intensifies, the therapeutic applications of cell and gene therapies diversify. From oncology to rare genetic disorders, the scope of these therapies widens, offering new avenues for medical intervention and treatment.
Collaborative Synergy
In an era of collaboration, industry players, research institutions, and regulatory bodies converge to amplify the impact of cell and gene therapy manufacturing market. Synergistic efforts pave the way for accelerated growth and groundbreaking discoveries.
A Renaissance in Healthcare Manufacturing
The cell and gene therapy manufacturing market stands at the cusp of a renaissance in healthcare manufacturing. The projected surge, backed by technological innovations, rising therapeutic efficacy, and regulatory support, heralds a new era of possibilities. As the sector navigates the growth corridor with a remarkable CAGR, the future promises not only financial prosperity but also transformative advancements in medical science.