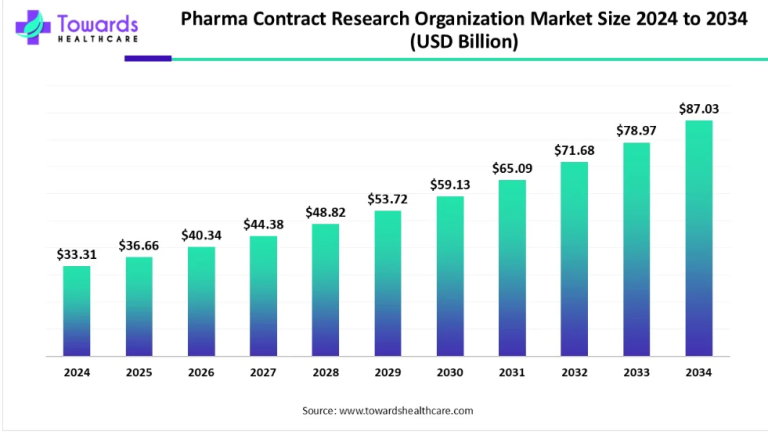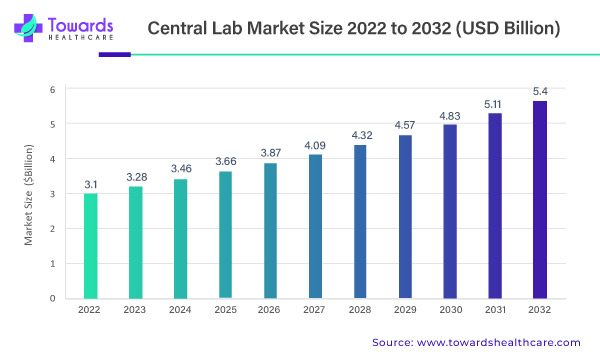
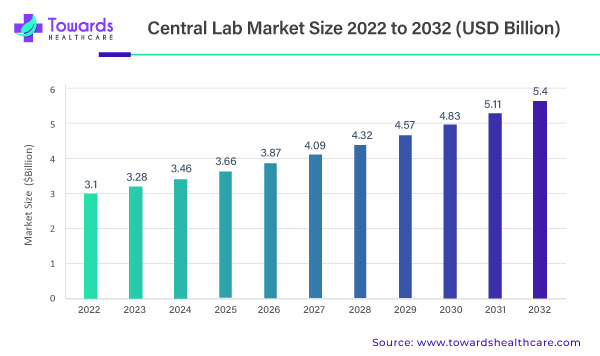
The global central lab market is poised for remarkable growth, projected to ascend from its 2022 valuation of USD 3.1 billion. Anticipating a steady 5.71% Compound Annual Growth Rate (CAGR) between 2023 and 2032, the market is forecasted to culminate in an impressive USD 5.4 billion. This meteoric rise is attributed to escalating investments in Research and Development (R&D) and a surging demand for clinical trials.
Fueling Innovation: Unleashing the Power of R&D Investment
In the dynamic landscape of healthcare, the surge in the central lab market is intricately tied to robust investments in Research and Development. As stakeholders channel resources into cutting-edge R&D initiatives, the market witnesses a transformative evolution, with advancements and breakthroughs driving unprecedented growth. This commitment to innovation not only propels scientific discovery but also positions central labs at the forefront of medical progress.
Meeting the Demand: Central Labs and the Clinical Trials Boom
Rising Demand for Clinical Trials
Central to the market’s upward trajectory is the escalating demand for clinical trials. As the healthcare landscape embraces a paradigm shift towards evidence-based practices, clinical trials play a pivotal role in validating new treatments and therapies. Central labs, equipped with state-of-the-art facilities and expertise, become integral partners in this process, fueling the demand for their services and contributing to the market’s exponential growth.
Pharmaceutical Giants are Accelerating the Growth in Central Lab Market
A central lab, also known as a central laboratory, is a specialized facility for conducting various laboratory tests and analyses. These labs are typically used in clinical trials, where they are responsible for performing a range of analyses on samples of blood, urine, tissue, and other materials collected from study participants. The goal of central labs is to provide consistent and reliable data across multiple study sites, which can then be used to evaluate the safety and effectiveness of new drugs, medical devices, or other healthcare interventions.
Central labs typically use advanced laboratory equipment and technologies, such as automated sample processing systems, high-throughput screening systems, and sophisticated analytical instruments. They are staffed by trained technicians and scientists who are responsible for conducting the tests and interpreting the results.
In addition to clinical trials, central labs may also be used for other types of laboratory testing, such as diagnostic testing for infectious diseases or genetic testing for inherited conditions. These labs may be operated by private companies, academic institutions, or government agencies, and they may be located in a variety of settings, including hospitals, research centers, and specialized laboratory facilities.
North America Dominates, Asia Pacific Emerges
The central lab market is witnessing growth globally, with North America leading the market, followed by Europe and the Asia Pacific. The North American market is driven by factors such as the increasing number of clinical trials, the presence of key players, and a favorable regulatory environment. The presence of leading pharmaceutical and biotechnology companies in the region is also contributing to market growth. North America was the highest contributor to this market, with 39.84%revenue share in 2022, and is anticipated to reach 36.95% revenue share by 2032, registering a CAGR of 4.7% (2023-2032).
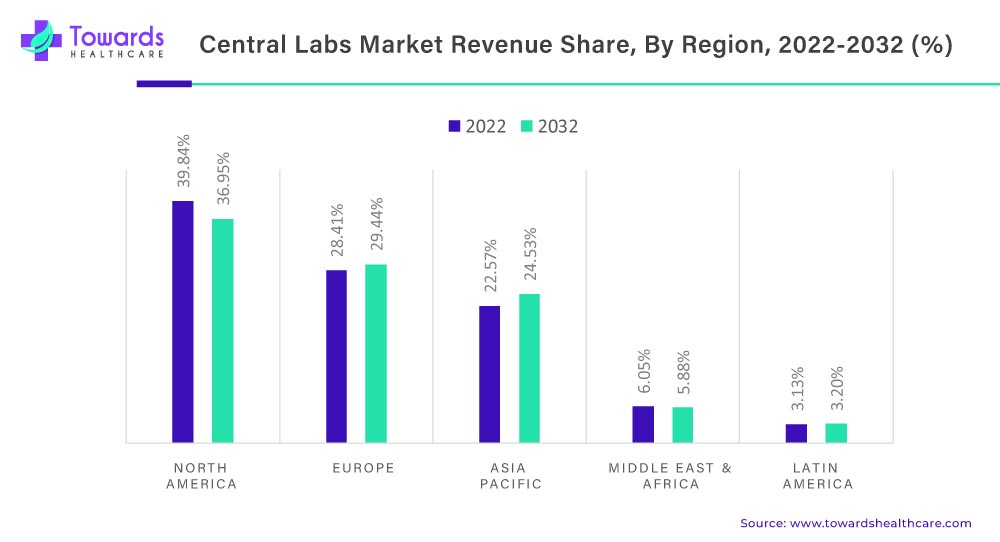
On the other hand, the Asia Pacific region is expected to emerge as a significant market for central lab market services, owing to the increasing number of clinical trials in the region, growing healthcare infrastructure, and rising investments by pharmaceutical and biotechnology companies. The Asia Pacific market is expected to witness significant growth in countries such as China, India, and Japan, major clinical research hubs.
Furthermore, the increasing prevalence of chronic diseases, the growing geriatric population, and the rising demand for personalized medicine are expected to drive market growth in the region. The market is also expected to witness growth in emerging economies such as Brazil and South Africa, owing to the increasing number of clinical trials and the growing focus on healthcare infrastructure development.
Biomarker Services: Advancing Precision Medicine for Better Patient Outcomes
Biomarker services play a crucial role in advancing precision medicine, a medical approach that tailors treatments to individual patients based on their genetic makeup, lifestyle, and other factors. Biomarkers are biological indicators that can be used to predict, diagnose, and monitor diseases, as well as to measure the effectiveness of treatments.
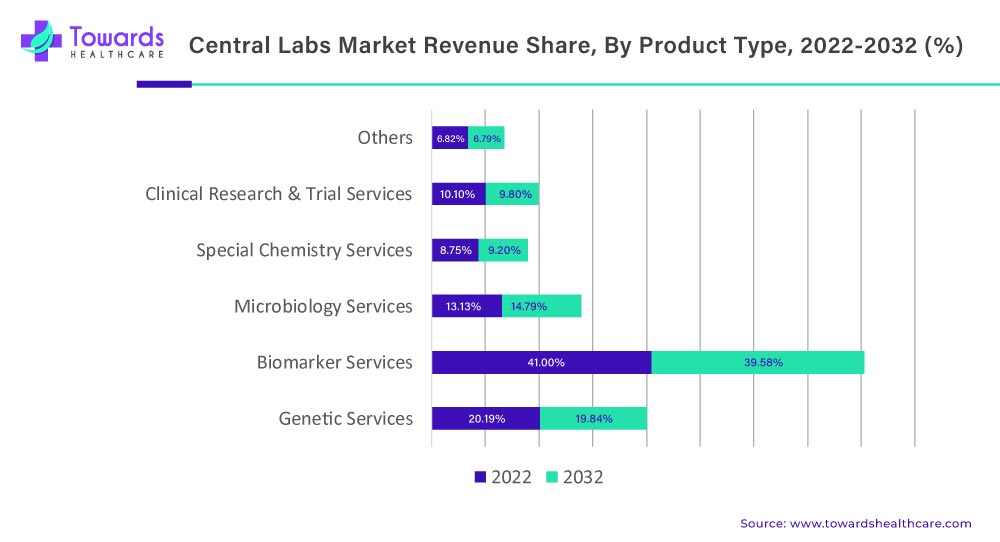
As of 2022, the biomarker services were the highest contributor to this market, with a 41.00% revenue share, and is anticipated to exhibit a 39.58% revenue share by 2032, registering a CAGR of 5.1% (2023 to 2032). While the microbiology services segment is projected to witness the fastest growth with a CAGR of 6.7% (2023-2032).
Central labs, which provide clinical trial testing services, have been increasingly incorporating biomarker services into their offerings. These services can include the development of biomarker assays, sample analysis, and data interpretation. By providing these services, central labs can help pharmaceutical and biotech companies identify the right patient populations for clinical trials and better understand the efficacy and safety of their drugs.
Biomarker services have also been instrumental in the development of new treatments for diseases, including cancer. For example, biomarkers can help identify which patients are most likely to respond to certain cancer treatments, allowing for more targeted therapies and better patient outcomes. Thus, the incorporation of biomarker services into central labs’ offerings has helped drive growth in the central lab market by increasing the demand for more advanced and personalized clinical trial testing services.
Pharma Dominance Fuels Expansion of Central Lab Market Services
Pharmaceutical dominance has fueled the expansion of central lab market services in recent years. With the increasing number of clinical trials and research activities conducted by pharmaceutical companies, the demand for central lab market services has grown significantly.
Central labs play a crucial role in clinical trials, providing accurate and reliable testing services for the evaluation of drug efficacy and safety. As the pharmaceutical industry continues to grow, so does the demand for central lab market services, driving market expansion.
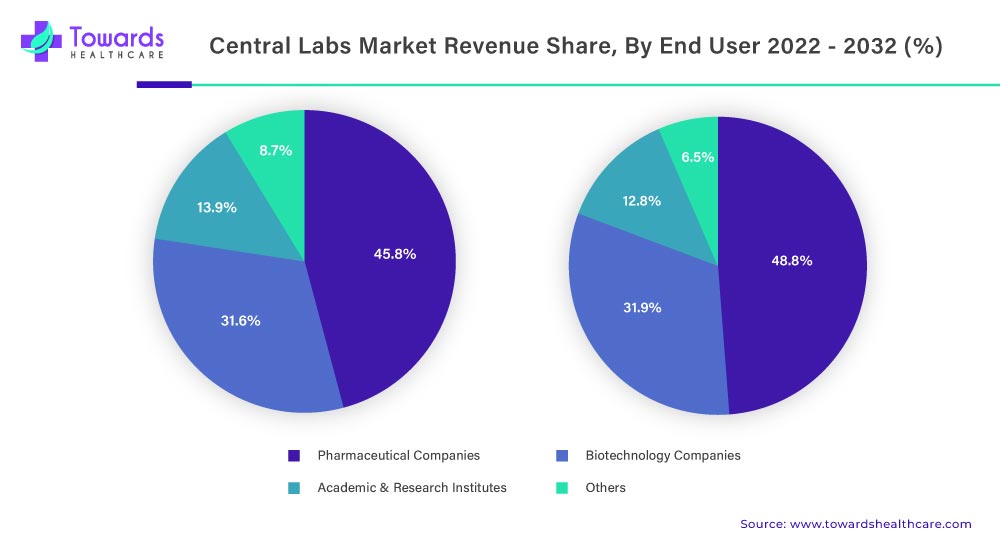
Pharmaceutical companies accounted for the largest revenue shareholder by end-user, in 2022 with around 45.8% market share in the central lab market. And are projected to register the highest growth over the forecast period with a CAGR of 6.1% (2022-2032).
In addition, the rising trend of personalized medicine has also contributed to the growth of central lab market services. Personalized medicine relies on the use of biomarkers to identify patients who are likely to respond to a particular treatment. Central labs provide biomarker testing services that enable clinicians to make more informed treatment decisions, leading to better patient outcomes.
Furthermore, the increasing prevalence of chronic diseases such as cancer and cardiovascular diseases has also driven the demand for central lab market services. These diseases require continuous monitoring of biomarkers and other laboratory parameters, which can be provided by central labs. The pharmaceutical industry’s dominance, the rising trend of personalized medicine, and the increasing prevalence of chronic diseases are the key drivers fueling the expansion of central lab market services.
Trials on the Rise: Meeting the Growing Demand for Clinical Research
The central lab market has been growing steadily in recent years, driven by a range of factors such as increasing demand for clinical research, advancements in technology, and the need for better patient outcomes. In particular, the growing number of clinical trials has been a major driving force behind the expansion of the central lab market. As the pharmaceutical industry continues to develop new drugs and therapies, the demand for clinical trials is expected to increase further. Central lab market play a critical role in supporting these trials by providing a range of testing and analysis services, including sample testing, biomarker analysis, and data management.
In addition, advancements in technology have made it easier and more efficient for central labs to handle large volumes of data and samples. This has led to increased productivity and faster turnaround times, which are important factors in the success of clinical trials. Thus, the growth of the central lab market is closely linked to the pharmaceutical industry and the demand for clinical research. As the industry continues to innovate and develop new treatments, the importance of central labs in supporting clinical trials is only expected to increase.
R&D Investment Booms: Propelling the Growth of the Market
The central labs market is experiencing significant growth, with one of the driving factors being the increase in R&D investment. As pharmaceutical companies seek to develop more effective drugs and therapies, they are investing more in clinical trials, which require the services of central labs. For instance, IQVIA spent around $160 billion on outsourced R&D in biopharmaceutical expenditure on drug development, in 2022.
The growing investment in R&D is not limited to pharmaceutical companies, as academic institutions, government organizations, and biotech startups are also increasing their research budgets. This trend is expected to continue, driving demand for central lab market services in the coming years. To meet the growing demand for central lab services, many companies are expanding their central lab facilities.
Additionally, the rise of precision medicine and personalized healthcare is fueling the need for advanced diagnostics and biomarker testing, which are often conducted by central labs. These factors are further driving the growth of the central lab market.
The increasing demand for precision medicine and personalized healthcare is a major driving force behind the growth of the central lab market. With the aim of developing targeted treatments that cater to the specific needs of individual patients, more sophisticated diagnostics and biomarker testing are needed, which in turn require the expertise of clinical trials. This has resulted in the increased use of biomarkers to identify specific diseases and to stratify patient populations for clinical trials.
Breaking Barriers: Strategies for Boosting Central Lab Services Growth Despite Time and Training Hurdles
Central labs are time-consuming because they involve multiple steps in the process of sample collection, transportation, analysis, and reporting. The market is also experiencing challenges due to the lack of skilled professionals. There are several ways to overcome these obstacles.
Automation can significantly reduce the time and effort required for laboratory testing, allowing central labs to process more samples in a shorter amount of time. This can help reduce turnaround times and improve overall efficiency, boosting customer satisfaction and driving market growth.
In addition, to ensure that central lab staff are equipped with the necessary skills and knowledge, it is essential to invest in training and education programs. By expanding these programs, central labs can improve the quality of their services and gain a competitive edge in the market.
Furthermore, emerging markets such as Asia Pacific and Latin America offer significant growth opportunities for the central lab market. By expanding into these regions, central labs can tap into new customer bases and drive market growth. Adopting innovative technologies such as digital pathology and next-generation sequencing can help central labs improve the accuracy and speed of their testing processes. This can help boost customer confidence and drive market growth.
Moreover, by offering value-added services such as consulting, data analysis, and clinical trial management, central labs can differentiate themselves from competitors and provide additional value to customers. This can help boost customer retention and drive market growth.
Navigating Central Lab Market Logistics: Overcoming Clinical Trial Challenges
Central laboratory logistics can present several challenges in the context of clinical trials. These challenges can arise due to several factors, including the need to handle a large volume of patient samples, the requirement for a standardized sample collection process across multiple study sites, and the need for timely and accurate delivery of samples to the central laboratory. One of the main challenges in central lab market logistics is ensuring the quality and integrity of samples during transportation. Samples must be transported under appropriate conditions to prevent degradation and maintain their stability. This requires specialized packaging, temperature monitoring, and transportation protocols to ensure that samples are delivered to the central laboratory in optimal condition.
Another challenge is ensuring the timely delivery of samples to the central laboratory. Delayed or lost shipments can result in compromised sample quality, inaccurate test results, and delays in the clinical trial timeline. This requires careful coordination between the study sites, clinical research organizations (CROs), and logistics providers to ensure that samples are collected, shipped, and received in a timely and efficient manner.
Additionally, there can be challenges related to data management and communication between study sites and the central laboratory. This requires a robust information technology infrastructure and effective communication channels to ensure that data and results are transmitted accurately and securely. Thus, successful navigation of central lab market logistics requires a combination of specialized expertise, effective communication, and careful planning and execution to ensure that clinical trial timelines and objectives are met.
Navigating Challenges, Seizing Opportunities
Technology Integration: A Strategic Imperative
With the rising complexity of clinical trials, central labs are faced with the challenge of integrating advanced technologies. Embracing automation, data analytics, and other technological innovations becomes not just a necessity but a strategic imperative. Labs that successfully navigate this integration emerge as leaders, offering efficient and reliable services that cater to the evolving needs of the healthcare industry.
Regulatory Landscape: Adapting for Success
In the dynamic world of clinical trials, the regulatory landscape is ever-evolving. Central labs must proactively adapt to changing regulations, ensuring compliance and reliability in their operations. Navigating this intricate terrain becomes a hallmark of success, positioning central labs as trusted partners in the journey towards groundbreaking medical advancements.
Virtual Reporting Revolution: Automation Driving Revenue Opportunities for Clinical Research
The virtual reporting revolution is transforming the clinical research industry by providing more efficient, accurate, and cost-effective solutions. Automation is driving revenue opportunities for clinical research by streamlining data management, reducing turnaround time, and increasing productivity. Virtual reporting solutions leverage artificial intelligence, machine learning, and natural language processing to automate data extraction, processing, and analysis. This eliminates the need for manual data entry, reduces errors, and improves the accuracy and speed of data reporting. Virtual reporting solutions also enable real-time data analysis, making it easier to identify trends, outliers, and potential issues, thereby improving decision-making.
In addition, the adoption of virtual reporting solutions is expected to drive revenue opportunities for the clinical research industry, as it allows companies to manage large data volumes more efficiently and effectively. It also improves the accuracy and speed of data processing and reporting, which can help companies bring products to market faster and with greater confidence.
Furthermore, virtual reporting solutions can be integrated with other systems, such as electronic data capture (EDC) and clinical trial management systems (CTMS), further streamlining the clinical trial process. This integration can improve data quality, reduce costs, and enhance collaboration among stakeholders. Moreover, the adoption of virtual reporting solutions is expected to drive revenue opportunities for the clinical research industry by improving the efficiency, accuracy, and speed of data reporting, enabling real-time data analysis, and integrating with other systems to streamline the clinical trial process.
A Future Defined by Collaboration and Innovation
In conclusion, the global central lab markets trajectory is marked by collaboration, innovation, and a steadfast commitment to advancing healthcare. As investments in R&D surge and the demand for clinical trials intensifies, central labs find themselves at the epicenter of this transformative journey. Navigating challenges, seizing opportunities, and embracing technological advancements, central labs emerge as beacons of progress, shaping a future where healthcare knows no bounds.