Epinephrine Market Growth and Key Drivers
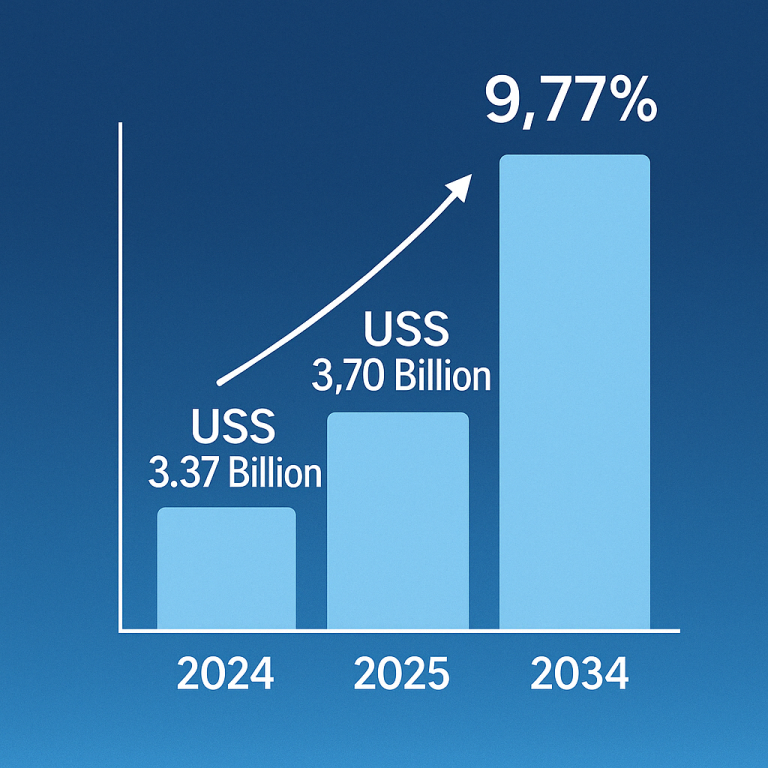
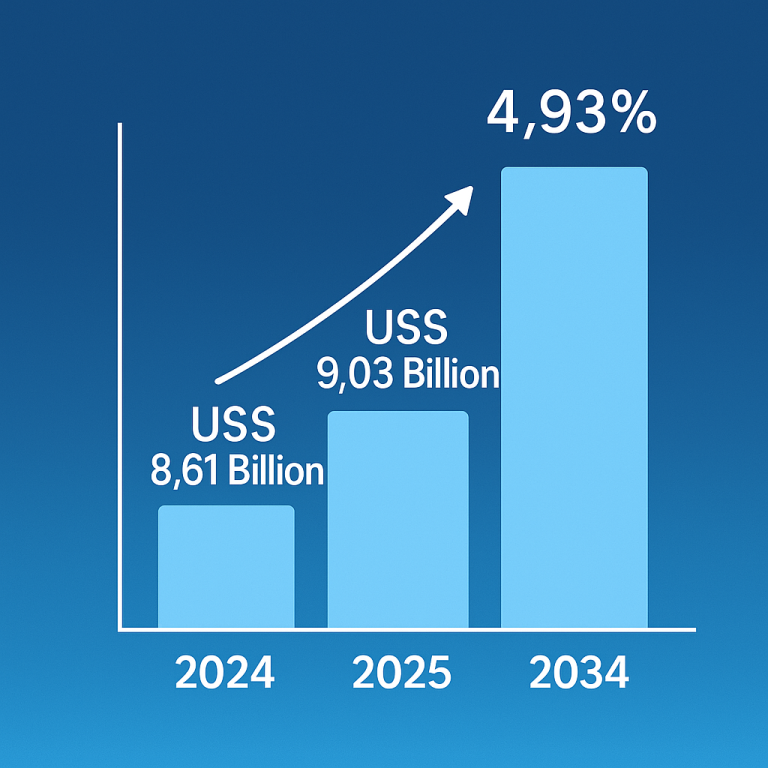
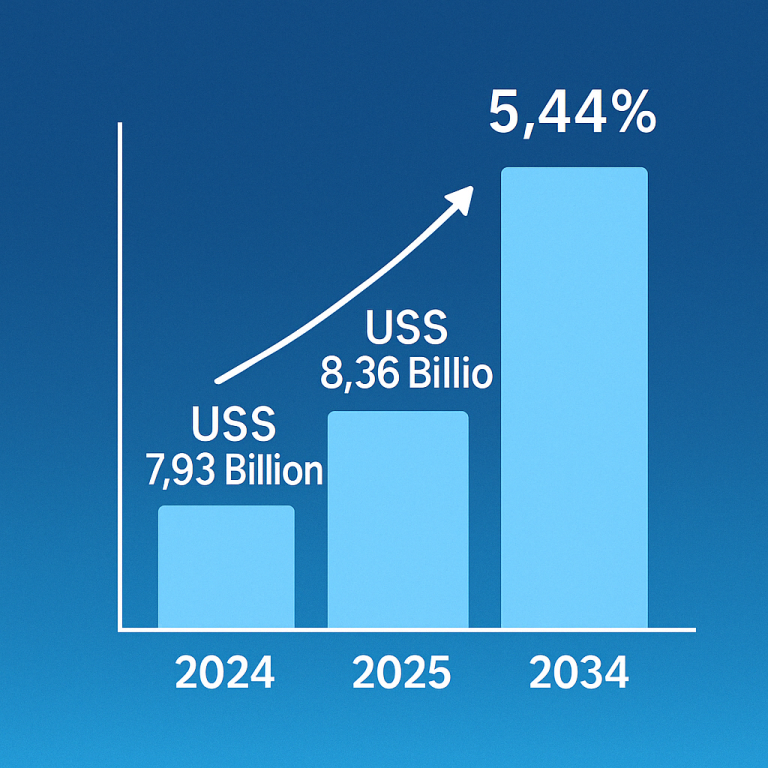
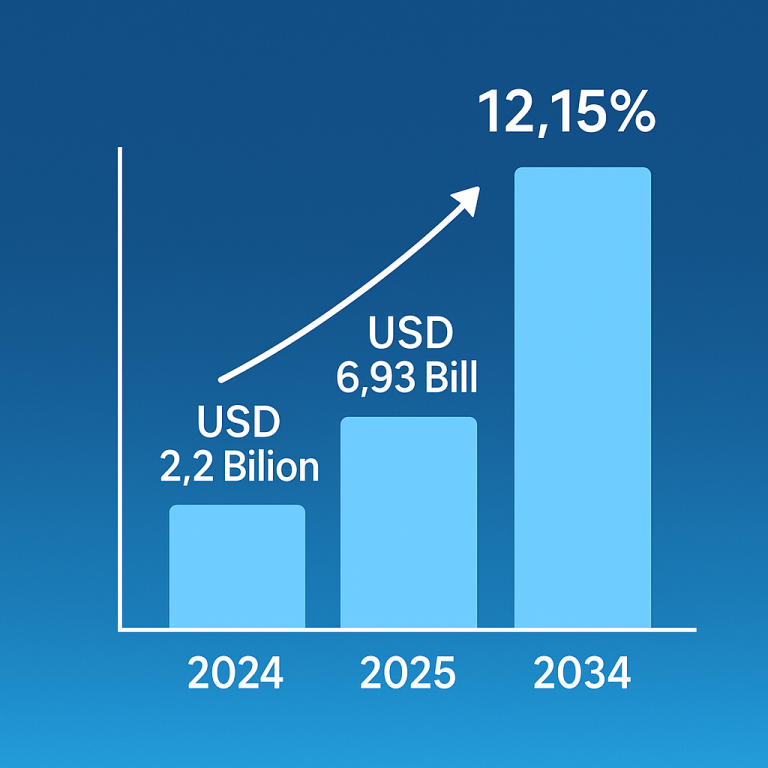
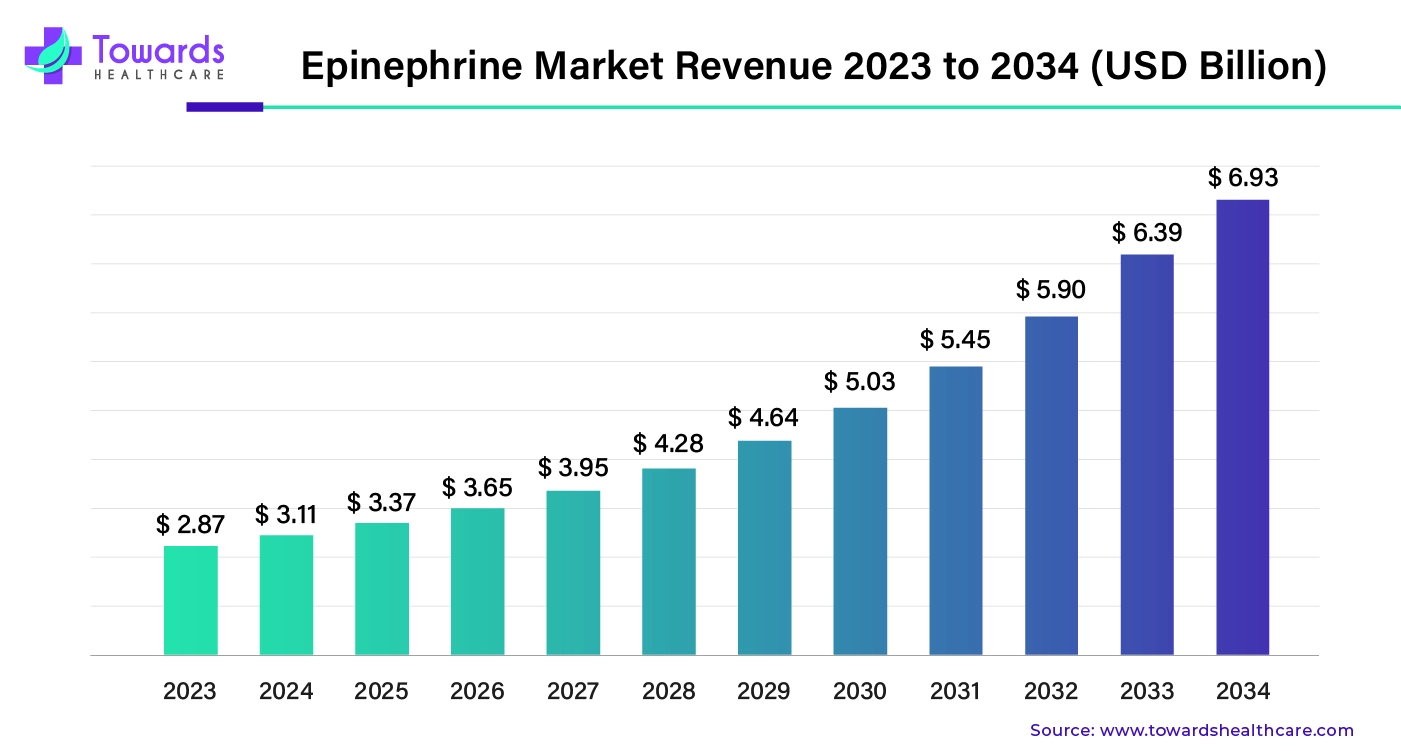
The global epinephrine market was valued at approximately $2.87 billion in 2023 and is expected to reach $6.93 billion by 2034, growing at a compound annual growth rate (CAGR) of 8.34% from 2024 to 2034.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5237
Key Insights into the Epinephrine Market
- North America held the largest market share, accounting for 36% of the global market in 2023.
- The Asia-Pacific region is expected to experience the fastest growth, with a projected compound annual growth rate (CAGR) of 7.49% during the forecast period.
- The auto-injector product segment represented a significant portion of the market, making up 78% of the total share in 2023.
- The prefilled syringes segment is anticipated to grow the most rapidly in the coming years.
- Anaphylaxis was the leading application in the epinephrine market, holding a 60% share in 2023.
- The cardiac arrest segment is predicted to see the fastest growth over the forecast period.
- Retail pharmacies led the global distribution channels, contributing to 41% of the market share in 2023.
- The online pharmacies segment is expected to experience the fastest growth between 2023 and 2034.
Recent Announcements and Developments in the Epinephrine Market
Sung Poblete, PhD, RN, CEO of Food Allergy Research & Education, praised the approval of the epinephrine nasal spray (NEFFY) in Europe, highlighting the benefits of a needle-free delivery system for the food allergy community. With over 33 million people in the U.S. affected by food allergies, NEFFY’s approval in the U.S. is eagerly anticipated as it offers a potentially life-saving treatment.
In recent market developments:
- In August 2024, AptarGroup, Inc. received approval for its Unidose Liquid System, a nasal drug delivery system designed to administer NEFFY, offering an effective option for patients experiencing severe allergic reactions.
- In December 2023, Nasus Pharma Ltd shared positive results from its Phase 2 clinical trial of FMXIN002, an intranasal epinephrine spray device. The trial confirmed the device’s safety and effectiveness in treating severe allergic reactions caused by food, medications, and insect stings.
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/epinephrine-market-sizing
Access exclusive insight now @ https://www.towardshealthcare.com/price/5237
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare