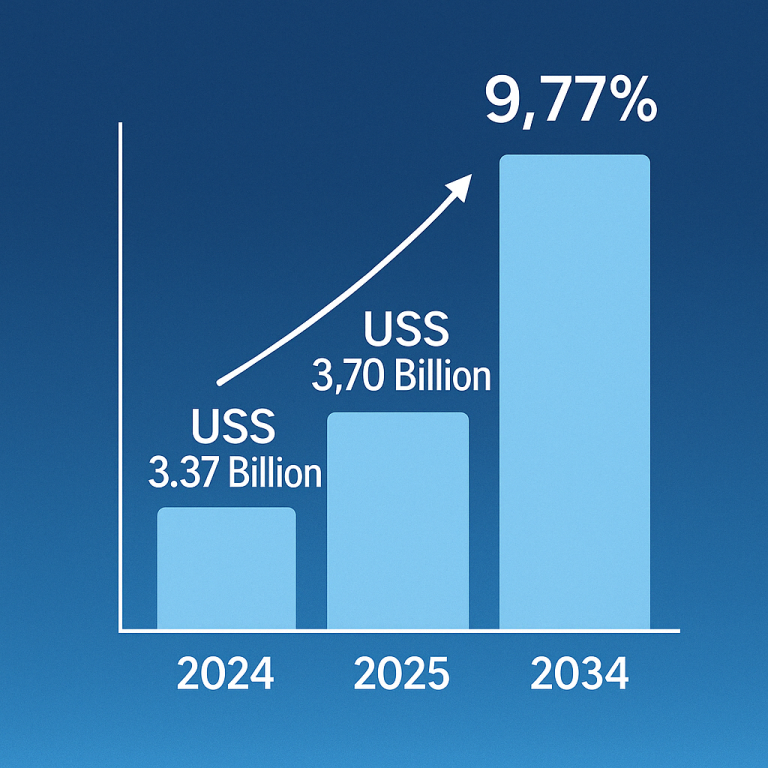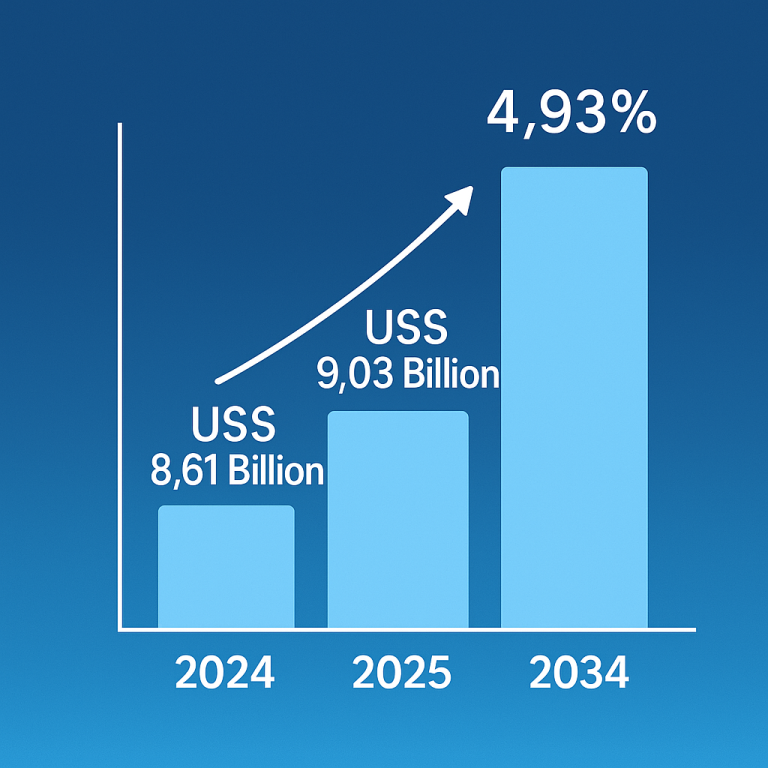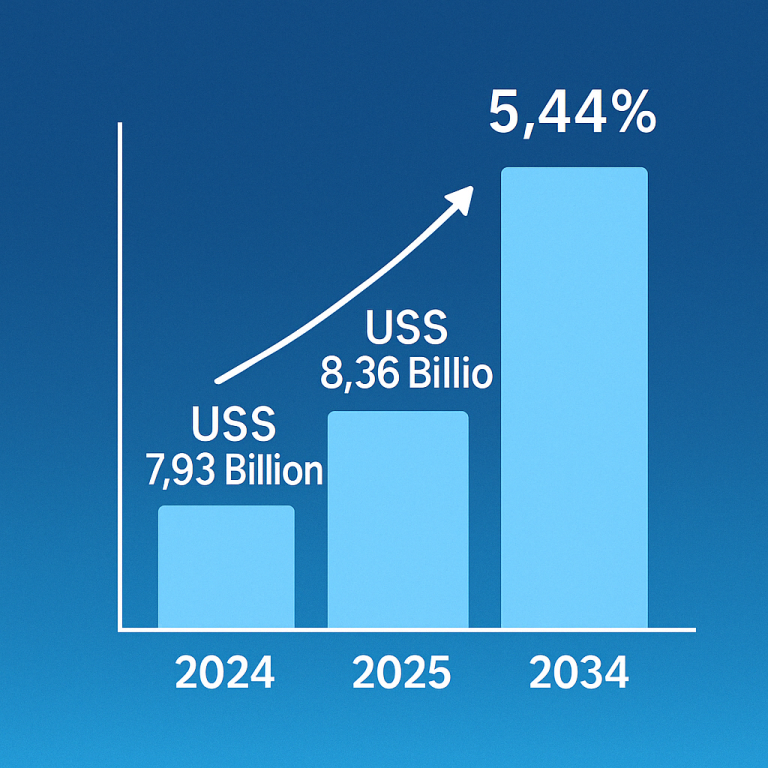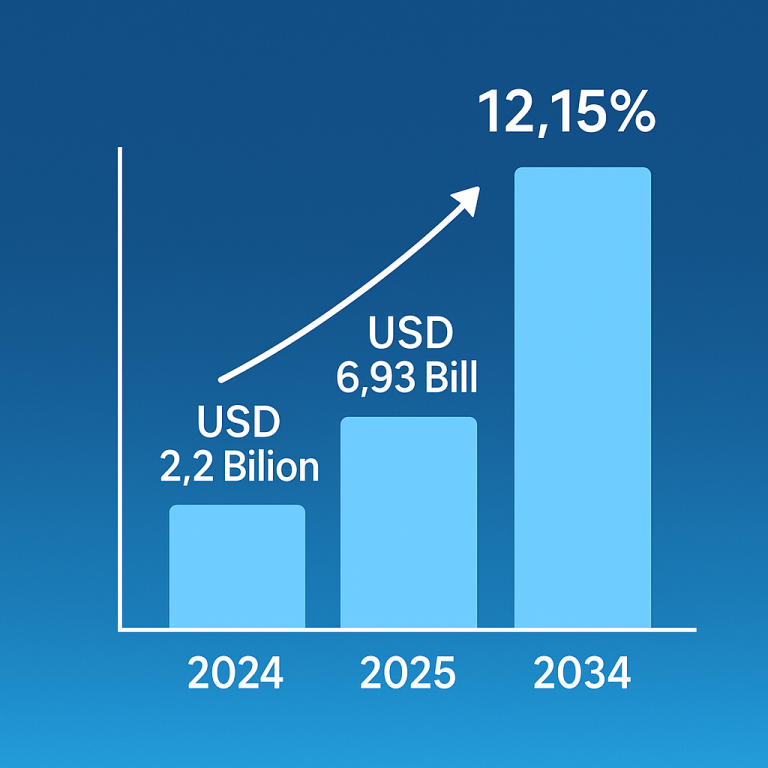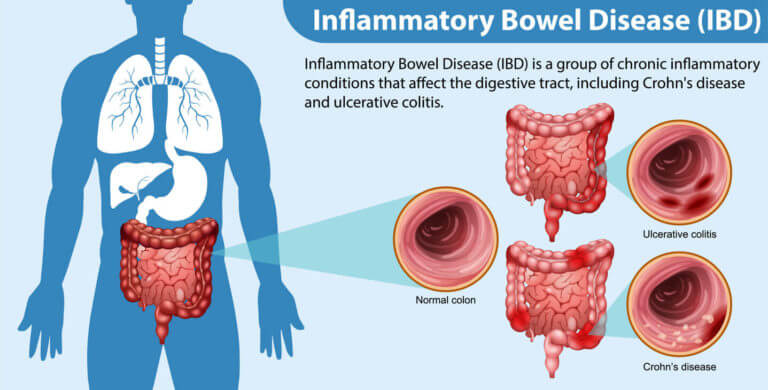
Inflammatory bowel disease (IBD), comprising Crohn’s disease and ulcerative colitis, is on the rise globally, posing significant challenges for healthcare and affecting millions. This increase may stem from factors like the Western diet, particularly rich in processed foods and harmful fats, which could disrupt gut bacteria and immune responses. For instance, over 0.7% of Americans are affected by IBD, with rates projected to climb.
Cigarette smoking, especially linked to Crohn’s disease due to nicotine’s impact on the immune system, is another factor. Sedentary lifestyles are also implicated, with regular physical activity potentially mitigating IBD risk by regulating immune function. Urban areas tend to see higher IBD rates, possibly due to environmental or lifestyle factors associated with city living.
Both Crohn’s disease and ulcerative colitis exhibit increasing prevalence trends. In Canada, anticipated figures for 2023 predict 825 cases per 100,000 individuals, translating to 322,600 Canadians living with IBD. By 2035, this could rise to 1.08% of the population, equating to 470,000 affected individuals, with noticeable increases across all age groups.
Pediatric-onset IBD is a growing concern, with estimates showing a rise in cases among children. Forecasts suggest a continued increase, contrasting with steady rates among adults and seniors. This divergence indicates differing contributing factors across age groups.
Overall, the rise of IBD underscores the need for proactive measures, including dietary adjustments, smoking cessation, increased physical activity, and targeted interventions tailored to different age demographics.
Download a short version of this report @ https://www.towardshealthcare.com/personalized-scope/5149
Table of Contents
ToggleThe GEM Project
The GEM Project, led by Crohn’s and Colitis Canada, is a significant endeavor aimed at advancing treatments for ulcerative colitis and Crohn’s disease, ultimately improving the lives of patients. As one of the largest health charity funders globally, they have invested over $145 million in research since 1974, driving notable progress in genetics, gut microbes, inflammation, and cell repair. Their contributions lay the groundwork for innovative therapies while also offering essential patient programs, services, advocacy, and awareness initiatives.
In 2023, the GEM project, in collaboration with the National Institute of Health, made a breakthrough by uncovering crucial factors influencing the development of Inflammatory Bowel Disease (IBD). Their research revealed significant differences in gut bacteria composition between individuals who develop Crohn’s disease and those who remain healthy for extended periods before onset.
Among the recent research programs funded by the GEM project are initiatives focused on understanding various aspects of Crohn’s disease and ulcerative colitis. These projects include Jennifer Jones’ investigation into how proteins protect the gut from harmful bacteria at Dalhousie University, Farhad Peerani’s exploration of immune mechanisms underlying ulcerative colitis in the elderly at the University of Alberta, Dana Philpott’s study on the connection between Crohn’s disease and Type-2 diabetes at the University of Toronto, and David Lohnes’ efforts to comprehend the causes of ulcerative colitis using a novel lab model at the University of Ottawa.
These endeavors underscore the commitment of the GEM Project and its collaborators to advancing knowledge, developing effective treatments, and ultimately improving outcomes for individuals living with Crohn’s disease and ulcerative colitis.
Government Initiatives in Finding IBD Cures, Augments the Market Growth
Government initiatives aimed at finding cures for inflammatory bowel diseases (IBD) are driving market growth. Currently, IBD lacks definitive treatments, with management of symptoms being the primary approach. This presents a significant growth opportunity in the field. Government agencies and various non-profit organizations are heavily investing in research endeavors to explore innovative treatment options for conditions like Crohn’s disease and ulcerative colitis.
For example:
- In March 2024, the Crohn’s & Colitis Foundation allocated funding to three firms as part of its IBD Ventures program’s 2023 financing round. This program focuses on accelerating the development of product-oriented research and development projects with the potential to enhance remission rates and improve the quality of life for individuals with IBD.
- In March 2024, Crohn’s & Colitis UK introduced a novel medication tool aimed at better understanding potential treatment alternatives that align with patient needs.
- In February 2024, a notable breakthrough occurred in the treatment of Inflammatory Bowel Disease (IBD). A collaborative effort between EnLiSense CCM, the Icahn School of Medicine at Mount Sinai, and funding from the Crohn’s & Colitis Foundation yielded promising results for a unique monitoring device called IBD Aware. This wearable device utilizes sweat-sensing technology and could potentially become the first solution for continuous, non-invasive monitoring of IBD. The successful completion of two critical trials in February 2024 underscores the significant potential of IBD Aware, offering hope for a more accessible and effective method for monitoring the disease.
Advancements in Crohn’s Disease Management: Innovations, Approvals, and Collaborations
In March 2024, Northwestern University researchers achieved a groundbreaking milestone with the invention of the first wireless, implantable temperature sensor, heralding a significant advancement in the management of Crohn’s disease. This innovative technology promises continuous, real-time monitoring of inflammation in patients, potentially revolutionizing the early detection of flare-ups. With timely intervention, clinicians could mitigate the long-term damage associated with these inflammatory episodes.
Furthermore, the approval of Rinvoq (upadacitinib) by the European Commission (EC) in April 2023 marked a pivotal moment in Crohn’s disease treatment, particularly for AbbVie. As the first oral Janus Kinase (JAK) inhibitor authorized in Europe for adult patients with moderately to highly active Crohn’s disease, Rinvoq offers a ray of hope for individuals who have not responded well to conventional or biologic therapies. The availability of two dosage options, an induction dose followed by tailored maintenance doses, underscores a personalized approach to treatment, offering renewed optimism for disease management.
In May 2023, a promising collaboration emerged in the fight against Crohn’s disease as Amgen and TScan Therapeutics, Inc. joined forces to leverage TScan’s TargetScan technology. This unique platform identifies the specific antigens recognized by T cells in Crohn’s disease patients, laying the foundation for the development of novel therapeutic strategies. The collaborative agreement, spanning multiple years, entails a substantial upfront payment of $30 million to TScan, with additional milestone-based payments exceeding $500 million. TScan stands to benefit from tiered, single-digit royalty payments tied to the commercial success of resulting therapies, incentivizing both organizations to advance this potentially transformative research.
In April 2022, the FDA unveiled draft guidelines aimed at steering sponsors in the clinical development of therapies for Crohn’s disease and ulcerative colitis. These recommendations, pertaining to drugs developed under specific sections of the Federal Food, Drug, and Cosmetic Act and the Public Health Service Act, as well as corresponding regulations, delineate the FDA’s stance on clinical trial parameters for addressing CD. Key aspects covered in this draft include trial population, design, efficacy, and safety considerations.
TNF Inhibitors in the Treatment of Inflammatory Bowel Disease
TNF inhibitors have revolutionized the management of Crohn’s disease, offering a safer and more efficacious alternative to existing treatments. Leading medications like Remicade (infliximab), Humira (adalimumab), and Enbrel (etanercept) have significantly impacted patient care for moderate to severe IBD. Their increasing utilization has fueled the growth of TNF inhibitors, effectively reducing inflammation and enhancing symptom control and overall quality of life for IBD patients. While some adverse effects may occur, TNF inhibitors generally boast better tolerability compared to other therapies.
Moreover, the expiry of patents for several TNF inhibitor drugs is opening doors for biosimilar equivalents, promising similar efficacy at a lower cost and potentially broadening access to the market. Despite the emergence of alternative classes like JAK inhibitors, TNF inhibitors are expected to maintain their prominence due to their established effectiveness.
The future trajectory of the IBD treatment market may involve personalized medicine tailored to individual patient responses and needs, potentially leveraging biomarkers to identify those who would benefit most from TNF inhibitors.
Thus, the TNF inhibitor segment of the IBD therapeutic market is firmly established and on the rise. Their proven efficacy, favorable safety profile, and extensive usage are likely to ensure their continued dominance in the foreseeable future. Nevertheless, the advent of biosimilars and ongoing exploration of novel treatments will undoubtedly shape the market landscape.
To own our research study instantly, Click here @ https://www.towardshealthcare.com/price/5149
Read More about Inflammatory Bowel Disease Treatment Market:
- Inflammatory Bowel Disease Treatment Market Size
- Inflammatory Bowel Disease Treatment Market Companies
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
About Us
Healthcare Web Wire is a premier subsidiary of Towards Healthcare, dedicated to providing comprehensive insights and information related to the healthcare industry. With a commitment to delivering accurate and timely updates, Healthcare Web Wire serves as a vital resource for professionals, enthusiasts, and stakeholders within the healthcare sector. Our platform serves as a central hub for the latest news, trends and developments shaping the healthcare landscape. Join us on Healthcare Web Wire and become part of a vibrant community dedicated to advancing healthcare knowledge and shaping the future of healthcare worldwide.
Explore the comprehensive statistics and insights on healthcare industry data and its associated segmentation: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare