The polyvinyl chloride (PVC) segment held a dominant presence in the IV bags market in 2023. PVC remains the most preferred material for manufacturing IV bags due to its reliability and cost-effectiveness. Medical-grade PVC-based tubular films are widely used as they are free from particulate contamination, micro-organisms, and pyrogens. Additionally, PVC IV bags offer transparency, flexibility, lightweight properties, and impact resistance. They are durable, water-tight, and can be sterilized using the autoclave method at 122°C. PVC IV bags are predominantly used for parenteral nutrition, packaging of rehydration solutions, and administering antibiotics and analgesics in hospital and veterinary settings. These attributes make PVC the material of choice for IV bag manufacturing.
Market Growth and Future Projections
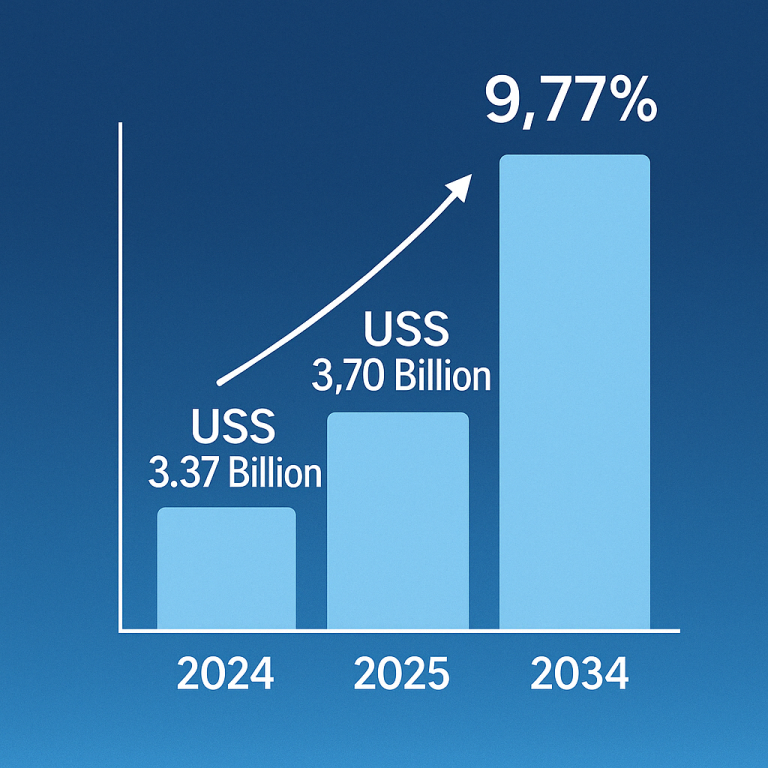
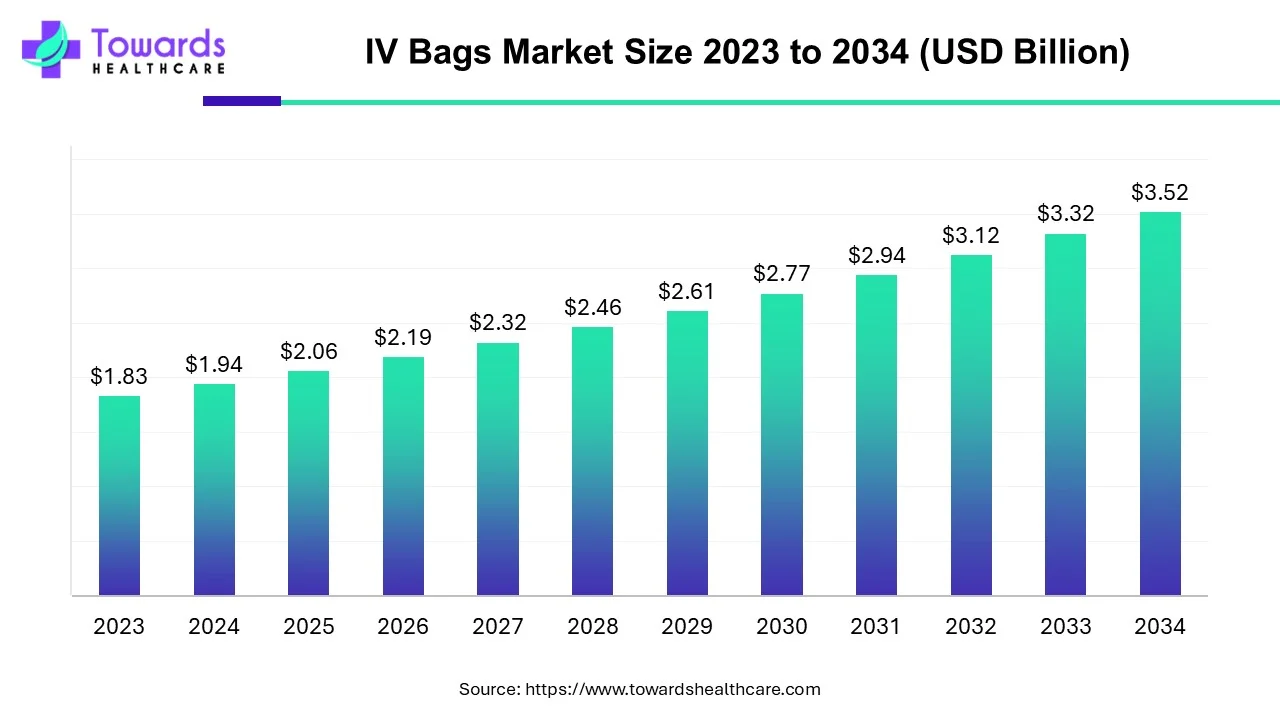
The IV bags market was estimated at US$ 1.94 billion in 2024, grew to USD 2.06 billion in 2025, and is projected to reach US$ 3.52 billion by 2034, rising at a compound annual growth rate (CAGR) of 6.14% from 2024 to 2034. The rising geriatric population, increasing acute and chronic disorders, growing adoption of intravenous therapies, and increasing healthcare investments are the key factors driving market expansion.
Get All the Details in Our Solution – Download Brochure @ https://www.towardshealthcare.com/download-brochure/5379
Significant Growth of the Polyethylene Segment
The polyethylene segment is predicted to witness significant growth in the IV bags market over the forecast period. The choice of IV bag material is crucial as it directly affects patient health. Polyethylene is the second-most preferred material for IV bags due to its lightweight, transparency, and durability, which facilitate easy handling, transportation, and storage. Furthermore, polyethylene is resistant to various chemicals, making it suitable for medical applications. Its ability to endure gamma irradiation without degradation ensures the integrity of IV bags, making it a viable alternative to PVC.
Dominance of the 250 ml to 500 ml Segment
The 250 ml to 500 ml segment registered its dominance in the global IV bags market in 2023. IV bag size selection in hospitals depends on patient condition and requirements. Smaller IV bag sizes are generally used for slower-rate infusions. For instance, a 250 ml IV bag is used for an infusion rate of 20 ml/hr, while a 500 ml IV bag is used for infusion rates ranging from 21 ml/hr to 40 ml/hr. A 500 ml IV drip typically takes 20 to 40 minutes to complete, depending on the flow rate. The average flow rate for a 250 ml IV bag is 42-62 drops per minute, while a 500 ml IV bag delivers 83-125 drops per minute. Medium-sized IV bags are preferred due to their ease of handling, lightweight properties, and compact storage requirements.
The Single Chamber Segment Leading the Market
The single chamber segment held the largest share of the IV bags market in 2023. Single chamber IV bags deliver a single IV fluid to the patient, ensuring consistent and controlled fluid administration. These bags minimize the risk of contamination by providing a sterile, latex-free environment. They are widely used to store and deliver a single type of medication or fluid, simplifying the infusion process and enhancing patient safety. Due to their user-friendly nature, single-chamber IV bags are highly preferred by healthcare professionals, ensuring efficiency and safety in patient care.
Parenteral Nutrition Segment Leading the Market
The parenteral nutrition segment led the global IV bags market in 2023. Parenteral nutrition involves delivering essential nutrients through an IV catheter directly into the bloodstream, particularly for patients suffering from malnutrition or digestive system issues due to surgery or chronic disorders. Increasing cases of colorectal cancer have further fueled the demand for IV fluids. Essential nutrients, including carbohydrates, fats, proteins, vitamins, and minerals, are administered through IV fluids, ensuring proper patient nourishment and recovery.
Key Industry Developments
- July 2024: Amneal Pharmaceuticals received US FDA approval for a New Drug Application (NDA) for its potassium phosphates in 0.9% sodium chloride injection IV ready-to-use bags. This injection provides phosphorus for adults and pediatric patients with hypophosphatemia.
- February 2024: B. Braun Medical, Inc. launched its Heparin Sodium 2,000 units in 0.9% Sodium Chloride Injection (1,000 ml). Manufactured using EXCEL IV Containers, the product is free from natural rubber latex, DEHP, and PVC, emphasizing patient and environmental safety.
- January 2024: Baxter International completed the first phase of its IV bag recycling program pilot in partnership with Northwestern Medicine in Chicago. The initiative successfully diverted over six tons (12,000 pounds) of PVC IV bag waste from landfills, repurposing the material for use in nonmedical products.
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/iv-bags-market-sizing
Invest in Our Premium Strategic Solution @ https://www.towardshealthcare.com/price/5379
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare