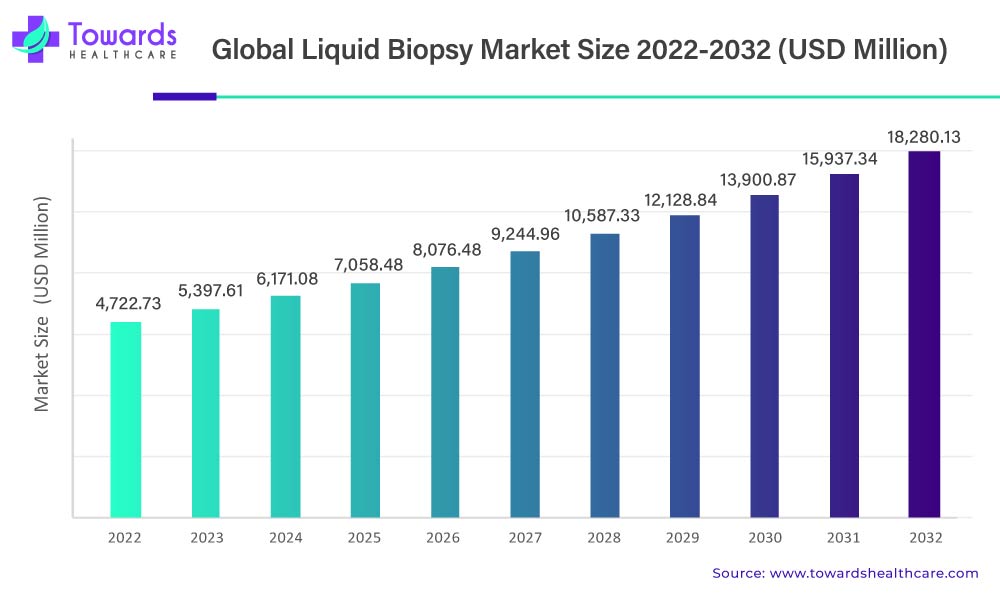
In the domain of advanced cancer diagnostics, the global liquid biopsy market is poised for remarkable growth. Commencing from its 2022 valuation of USD 4,722.73 million, the market is projected to undergo a robust Compound Annual Growth Rate (CAGR) of 14.5% during the period 2023-2032. By 2032, industry experts anticipate the market to achieve an estimated value of USD 18,280.13 million, propelled by transformative technological advancements in cancer diagnostics and the increasing preference for minimally invasive cancer diagnostic methods.
Propellors of Progress: Dynamics Shaping the Liquid Biopsy Market
1. Technological Advancements in Cancer Diagnostics:
At the core of the anticipated market surge is the wave of technological advancements revolutionizing cancer diagnostics. Liquid biopsy market, as an innovative approach, leverages cutting-edge technologies to detect and monitor cancer-related genetic alterations, driving the market’s expansion.
2. Rising Preference for Minimally Invasive Cancer Diagnostics:
The shift towards minimally invasive cancer diagnostics is a pivotal factor contributing to the market’s upward trajectory. Liquid biopsy market, requiring only a blood sample, offers a less invasive alternative to traditional tissue biopsies, aligning with the growing preference for patient-friendly diagnostic methods.
Technological Advancements in Genomic Analysis, and a Growing Preference for Personalized Medicine Fuel Market Growth
The liquid biopsy market refers to the market for non-invasive diagnostic tests that use a patient’s blood, urine, or other bodily fluids to detect cancer or other diseases. The liquid biopsy market is growing rapidly due to a number of factors, including the increasing incidence of cancer, advances in genomics and proteomics, and the need for non-invasive diagnostic tests.
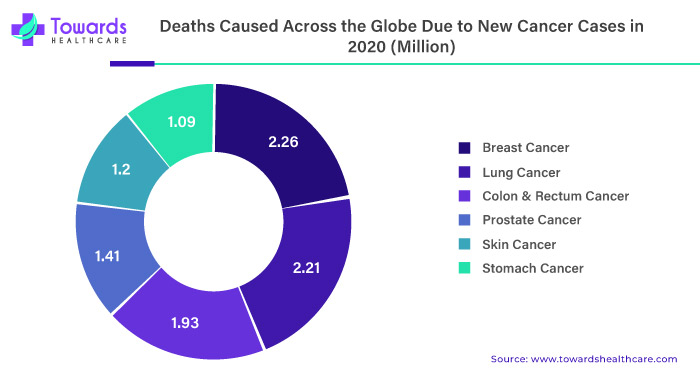
One of the significant drivers of the liquid biopsy market is the increasing incidence of cancer worldwide. According to the World Health Organization, cancer is the leading cause of death globally, accounting for an estimated 10 million deaths in 2020. Liquid biopsies offer a less invasive and more convenient way to detect cancer and monitor disease progression compared to traditional tissue biopsies, making them an attractive option for patients and healthcare providers.
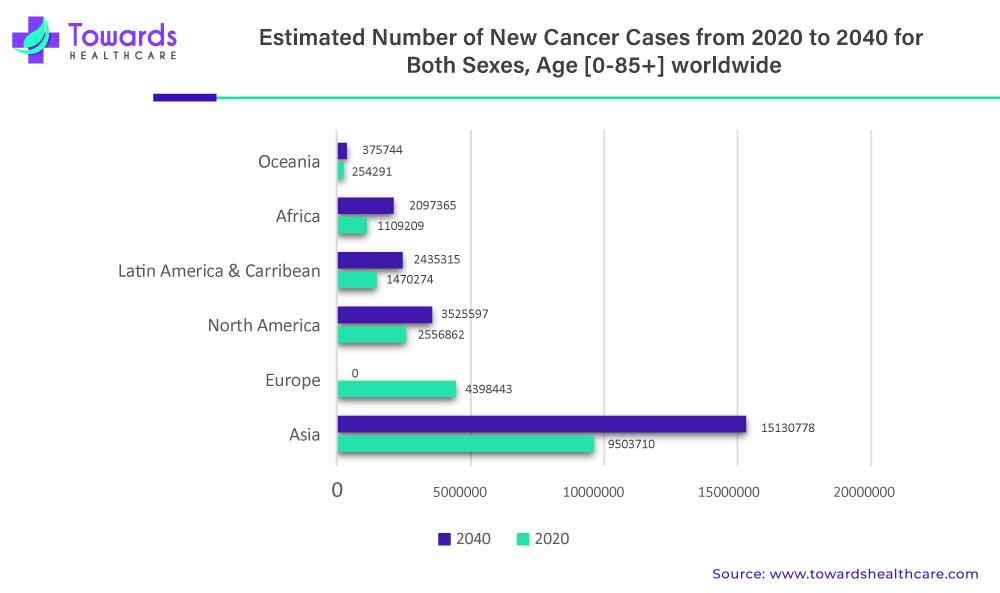
The advent of liquid biopsy market has connected targeted treatments and molecular diagnostic tests in a highly synergistic way. Liquid biopsy is a non-invasive diagnostic method that involves the analysis of various biomarkers in bodily fluids such as blood, urine, and saliva to detect the presence of cancer and other diseases. This method has gained increasing attention in recent years due to its potential to provide a less invasive and more accurate diagnosis and monitoring of cancer than a traditional tissue biopsy.
One of the latest trends in the liquid biopsy market is the development of multi-analyte liquid biopsy tests that can detect multiple types of biomarkers in a single test. These tests can improve the accuracy of cancer diagnosis and monitoring by providing a more comprehensive analysis of the disease. The integration of liquid biopsy with AI is another trend in the market. AI algorithms can analyze large amounts of data generated by liquid biopsy tests to identify patterns and biomarkers that may not be detectable by traditional analysis methods, improving the accuracy and precision of diagnosis and monitoring.
One of the main advantages of liquid biopsy market over traditional tissue biopsy is its non-invasive nature, which makes it a more comfortable and less risky procedure for patients. Another advantage is the ability to monitor disease progression and treatment response over time, which can help doctors make more informed decisions about treatment options. Liquid biopsy market has several potential applications, including cancer diagnosis, treatment monitoring, and early detection. Liquid biopsy can also detect other diseases, such as infectious, autoimmune, and cardiovascular diseases.
Advances in genomics and proteomics have also played a significant role in the growth of the liquid biopsy market. With the advent of next-generation sequencing (NGS) technologies and other advanced molecular techniques, it is now possible to detect and analyze cancer-specific mutations and biomarkers in a patient’s blood or urine. This has enabled the development of highly sensitive and specific liquid biopsy market tests that can detect cancer at early stages and monitor disease progression.
The need for non-invasive diagnostic tests has also contributed to the growth of this global market. Traditional tissue biopsies are invasive and often require hospitalization, anesthesia, and lengthy recovery times. In contrast, liquid biopsies are minimally invasive, can be performed in an outpatient setting, and often provide faster results, making them an attractive option for patients and healthcare providers.
In terms of applications, the liquid biopsy market is primarily driven by the oncology segment, which includes the detection and monitoring of cancer. However, liquid biopsies are also being developed for a range of other applications, including prenatal testing, infectious disease diagnosis, and organ transplant monitoring. Liquid biopsy market represents a promising new diagnostic approach that has the potential to significantly improve patient outcomes and reduce healthcare costs by enabling earlier detection and more accurate monitoring of cancer and other diseases. The market is projected to grow in the coming years as liquid biopsy market technologies become more widely adopted and new applications are developed.
Thus, the liquid biopsy market is expected to continue to grow rapidly in the coming years, driven by advances in genomics and proteomics, increasing incidences of cancer, and the need for non-invasive diagnostic tests. However, there are also challenges facing the market, including the lack of standardized testing protocols and regulatory oversight, as well as the high cost of liquid biopsy market tests.
AI to Drive Innovation in Liquid Biopsy Market
The U.S. National Library of Medicine studied the effect of liquid biopsy market using AI in triple-negative breast cancer, in May 2021.
Artificial intelligence has presented promising diagnostics to deliver incredible treatment outcomes. Several government and private organizations are engaged in conducting exhaustive research to study and advance the use of AI in liquid biopsy market. Additionally, several companies are developing liquid biopsy platforms that incorporate AI algorithms for data analysis. These platforms are designed to automate the liquid biopsy workflow, from sample preparation to data analysis, and provide clinicians with actionable insights in real time.
Furthermore, AI-driven liquid biopsy market analyses are helping identify specific mutations and biomarkers that are unique to an individual’s cancer, allowing for more personalized treatment approaches. AI algorithms potentially analyze liquid biopsy data to identify drug targets and predict treatment response.
How COVID-19 Pandemic Affected Liquid Biopsy Market
The COVID-19 pandemic has had both positive and negative impacts on the liquid biopsy market. The pandemic led to a delay in cancer screenings and diagnoses, as patients tend to hesitate to visit healthcare facilities due to the risk of infection. This resulted in lower demand for liquid biopsy tests, as fewer patients were being diagnosed with cancer and undergoing treatment. The pandemic disrupted global supply chains, which impacted the availability of liquid biopsy market tests and equipment.
This led to delays in testing and treatment for some patients during the pandemic. The negative impact was likely to be stronger in low- and middle-income countries with poor infrastructure, limited resources, scarcity of medical supplies & personal protective equipment, and shortage of healthcare providers & organized care teams, resulting in a lack of ability to provide & deliver critical care.
In addition, the greater need to utilize healthcare professionals and resources to address the pandemic resulted in the suspension of cancer screening programs for asymptomatic patients in several nations. This led to negative consequences, primarily delayed diagnoses.
The Lancet Oncology’s August 2020 data estimated a reduction in cancer survival rate, varying between 6.1–6.3% (esophageal, scenarios B and C) & 1.0–1.1% (breast, all scenarios) at one year after diagnosis, and between 6.4% (colorectal, scenario C) & 3.5% (lung, scenario A) at five years after diagnosis. In these cases, liquid biopsy provides an efficient alternative to traditional screening.
On the other hand, the pandemic also accelerated the adoption of liquid biopsy market testing in some cases. For example, liquid biopsy market tests had been used to monitor COVID-19 patients for the presence of viral RNA in their bloodstream, which could help predict disease severity and guide treatment decisions. Amid COVID-19, several companies adopted various strategic initiatives for providing safe and easy in-home access for liquid biopsy market tests.
For instance, in November 2020, NeoGenomics, Inc. launched a mobile phlebotomy service for liquid biopsy tests, including NeoLAB and InvisionFirst. The company offers its service through two phlebotomy companies, ExamOne and Metro Health Staffing LLC, for broad geographic coverage to ensure tests are performed efficiently.
Revolutionizing Cancer Diagnostics: How New Technologies are Changing the Game
In September 2022, Guardant Health, Inc. launched GaurdantINFINITY, a liquid biopsy assay for research purposes to hasten the development of new cancer therapies for patients.
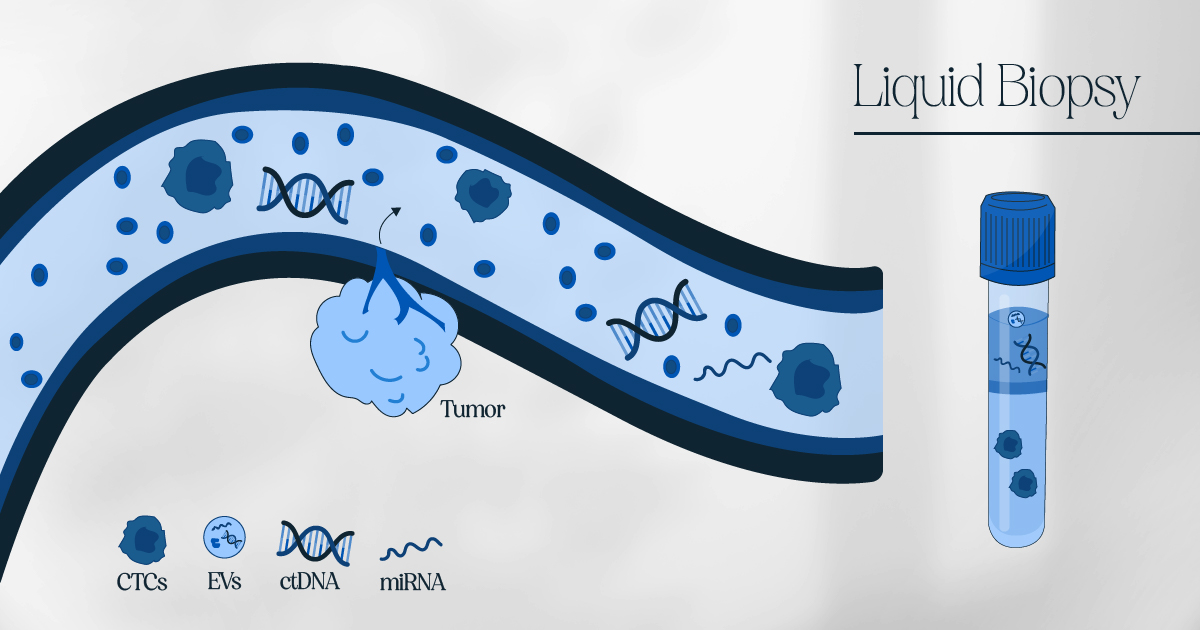
Liquid biopsy is a less invasive technique for identifying non-hematological cancers, and it has gained popularity due to technological advancements in ongoing research on circulating biomarkers. A liquid biopsy involves analyzing circulating tumor DNA, circulating tumor cells, exosomes, and extracellular vesicles, which have the potential to transform cancer management and treatment dynamics. Compared to traditional tumor biopsy, liquid biopsy is considered a viable option for real-time patient monitoring.
Compared to traditional tumor biopsy, liquid biopsy is considered a viable option for real-time patient monitoring. Comparing liquid biopsy to standard tumor biopsy, liquid biopsy is considered a viable option for real-time patient monitoring. It includes circulating tumor DNA, circulating tumor cells, exosomes, and extracellular vesicles that have the potential to significantly transform cancer management and treatment dynamics. Moreover, the market is expected to grow by providing precise and personalized treatment in the coming years.
Liquid biopsy is an advanced testing technology for detecting tumor-related genetic alteration. It has also been used to stratify tumors and provide appropriate precision oncology treatment. For instance, in October 2020, Foundation Medicine’s FoundationOne Liquid CDx received approval from the FDA to identify prostate and lung cancer in patients. These recent advancements, innovations, and expansions in the industry promoting liquid biopsy are driving the market’s growth.
Massive parallel or NGS is the fastest growing and evolving technology with increasing applications in various human diseases due to the advantages of liquid biopsy technologies. The increase in the alteration of molecules across multiple cancer types supports the usage of NGS in clinical settings, as it provides a cost- and time-effective way of detecting cancer. Tumor heterogeneity (evaluating different genes simultaneously) and the need for gene profiling has increased the use of liquid biopsy, which is expected to propel market growth.
Less is More: The Growing Popularity of Minimally Invasive Cancer Diagnostics
The rising prevalence of cancer is a significant driving factor for the growth of the liquid biopsy market. According to the World Health Organization (WHO), cancer is one of the leading causes of death globally, and it is estimated that approximately 10 million people die from cancer every year. Additionally, the incidence of cancer is increasing, primarily due to factors such as population growth, aging, and lifestyle changes. This has led to an increased demand for accurate and efficient cancer diagnostics and treatment options, including liquid biopsy.
Liquid biopsy offers several advantages over traditional tissue biopsy, such as less invasiveness, the ability to perform real-time monitoring, and the potential to identify genetic alterations that may not be detected by tissue biopsy. Liquid biopsy can also be used for early cancer detection and monitoring of treatment response, which can lead to improved patient outcomes. As a result, liquid biopsy has become an increasingly important tool in cancer diagnosis and treatment.
Liquid biopsy technologies have made considerable headway in recent years, showing a significant boom in adoption and clinical applications. Liquid biopsy is a rapidly evolving tool of precision oncology, enabling longitudinal monitoring and minimally invasive molecular diagnostics for treatment purposes. For managing advanced-stage lung cancer, quantifying and detecting circulating DNA are now commonly adopted as clinical practice.
Moreover, liquid biopsy technology is delivering a new source for cancer biomarkers and adding new dimensions to clinical trials. A liquid biopsy helps diagnose cancer at an early stage, identify resistance mechanisms of therapies, and customize treatment for disease detection, which is expected to contribute to market growth.
Although conventional tissue biopsies involve invasive methods for detecting tumor cells, liquid biopsies involve using peripheral blood, which is easily accessible, allowing for more common use, primarily in patients who cannot undergo surgery. As a result, it takes less time for treatment based on tumor detection, enhancing staff & resources efficiency, and is used to screen other diseases. It can also help avoid potential complications associated with conventional biopsies, removing the risk of tumor spread, severe bleeding, and injury to surrounding tissue. Thus, liquid biopsy is widely accepted and expected to drive market growth soon.
For various applications such as non-small-cell lung cancer, breast, prostate, colorectal, and ovarian cancer, liquid biopsy is used for diagnostic and screening purposes, making it an essential tool. After various studies and speculations, it has been derived that the liquid biopsy technique could provide an improved diagnosis outcome. Data shows that screening techniques should be used on high-risk patients with an ancestral cancer history. Currently, community practices and academic centers use liquid biopsy as their complementary technique compared to standard tissue biopsies, which is expected to drive the market globally.
Navigating the Diagnostic Landscape: Future Projections and Market Dynamics
1. Expanding Applications of Liquid Biopsy:
The versatility of liquid biopsy applications is expanding beyond its initial scope. From early cancer detection to treatment monitoring, liquid biopsy is becoming an indispensable tool in the diagnostic arsenal, contributing significantly to the market’s sustained growth.
2. Global Collaboration for Diagnostic Excellence:
Collaborations among global stakeholders are fostering an environment conducive to diagnostic excellence. Research partnerships, technological collaborations, and knowledge-sharing initiatives are driving innovation, positioning liquid biopsy at the forefront of cancer diagnostics.
Navigating the Challenges of Biopsy Procedures: Overcoming Restraints to Improve Cancer Diagnosis
Biopsy procedures have long been considered the gold standard for cancer diagnosis, but they are not without their challenges. One of the main challenges is the lower sensitivity of certain biopsy procedures, which can lead to false-negative results or inconclusive diagnoses. This can delay proper treatment and lead to poorer outcomes for patients.
The lower sensitivity of certain biopsy procedures is one of the restraints for the liquid biopsy market. While liquid biopsy offers many advantages over traditional tissue biopsy, such as being less invasive, faster, and allowing for real-time monitoring, it may not be suitable for all types of cancers and all stages of cancer. Some cancers, such as brain cancer, may not shed enough DNA or cells into the bloodstream for liquid biopsy to detect accurately. Moreover, a liquid biopsy may not be able to identify the precise location of cancer, which may be necessary for surgical planning.
Additionally, some liquid biopsy techniques, such as the analysis of circulating tumor DNA (ctDNA), may have a lower sensitivity compared to tissue biopsy, particularly in early-stage cancers or small tumors. False negatives or false positives may also occur due to technical issues, such as the degradation of the DNA during the collection or analysis process, or due to the presence of non-cancerous DNA mutations. These limitations may impact the accuracy of liquid biopsy results, and some patients may still require tissue biopsy to confirm a cancer diagnosis or treatment plan.
Therefore, the lower sensitivity of certain biopsy procedures remains a significant challenge for the liquid biopsy market. However, ongoing research and development in liquid biopsy technology and the identification of new biomarkers may address some of these limitations and improve the accuracy and reliability of liquid biopsy for cancer diagnosis and treatment monitoring. Furthermore, the growing demand for personalized and precision medicine may drive the development of liquid biopsy techniques that can detect a wider range of biomarkers and offer more comprehensive diagnostic and treatment options for cancer patients.
To address this challenge, researchers are exploring the use of liquid biopsy as a complementary tool to traditional biopsy procedures. Liquid biopsy involves the analysis of biomarkers such as circulating tumor DNA, circulating tumor cells, and exosomes in a patient’s blood or other bodily fluids. These biomarkers can provide valuable information about the presence and characteristics of cancer, even at early stages or in hard-to-reach locations.
Addressing Challenges: Strategic Approaches for Sustainable Growth
While the outlook for the global liquid biopsy market is promising, it’s crucial to address potential challenges. Regulatory considerations, standardization of procedures, and market competition are focal points where industry stakeholders are actively implementing strategies for sustained growth and continued market leadership.