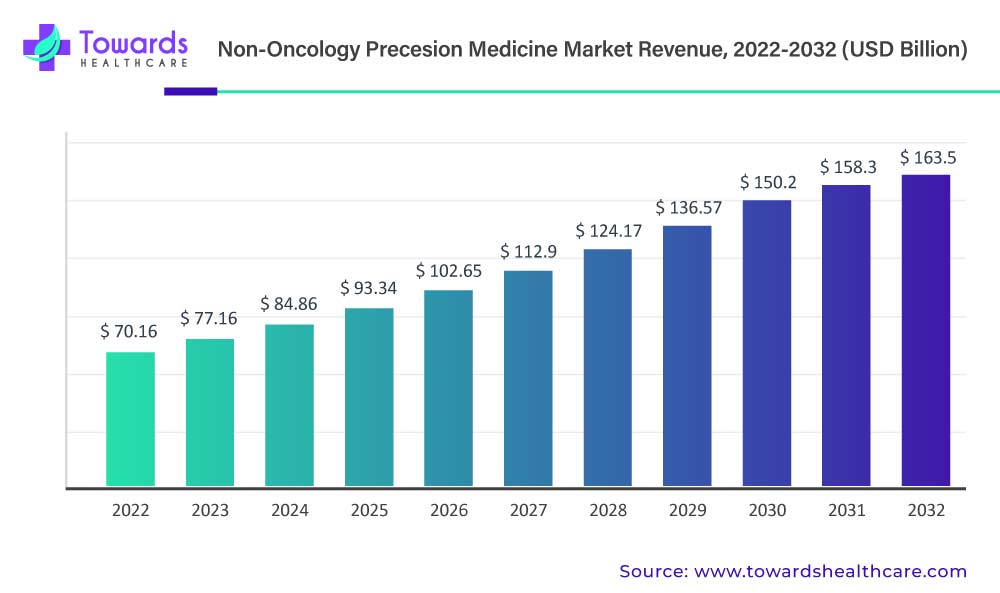
The non-oncology precision medicine market has not only thrived but soared to unprecedented heights, boasting a colossal size that stood at USD 70.16 billion in 2022. A testament to its vigor, this market is poised for even more substantial growth, with projections indicating a staggering climb to approximately USD 163.5 billion by 2032.
Pinnacle Achievements: Navigating the Landscape of Non-Oncology Precision Medicine Market
Rapid advancements in genomics and molecular diagnostics driving personalized treatment approaches in non-oncology conditions.
The non-oncology precision medicine market is a rapidly growing sector within the healthcare industry. Precision medicine aims to provide tailored treatment and preventive measures based on an individual’s genetic, environmental, and lifestyle factors. While precision medicine initially gained traction in the field of oncology, its applications are expanding to other disease areas.
The non-oncology precision medicine market is driven by several factors. First and foremost, advancements in genomics, proteomics, and other molecular diagnostic technologies have enabled the identification of specific biomarkers and genetic variations associated with various non-oncological diseases. This knowledge allows for more targeted and personalized approaches to treatment and prevention.
Non-oncology precision medicine market finds applications in diverse therapeutic areas, including cardiovascular diseases, neurology, infectious diseases, autoimmune disorders, and rare genetic conditions. By understanding an individual’s unique genetic makeup and disease risk factors, healthcare providers can design customized treatment plans and interventions. As precision medicine continues to evolve and gain acceptance in non-oncology areas, it is expected to revolutionize the healthcare industry by shifting from a one-size-fits-all approach to a more personalized and targeted approach to patient care.
The Role of Advancements in Genomic Technologies
Advancements in genomic technologies are a major driver for the non-oncology precision medicine market. Genomic sequencing, high-throughput screening, and other molecular diagnostic techniques have significantly enhanced our understanding of the genetic basis of non-oncological diseases.
Genomic sequencing technologies, such as next-generation sequencing (NGS), have become more accessible and cost-effective, enabling the analysis of an individual’s entire genome or specific genetic regions associated with disease susceptibility. In August 2022, Mount Sinai Health System initiated a genetic sequencing project in collaboration with the Regeneron Genetics Center. This project aims to enroll 1 million patients over a span of five years to advance precision medicine and enhance patient care. This wealth of genetic information allows healthcare providers to identify genetic variations, mutations, and biomarkers that contribute to the development and progression of non-oncological diseases.
In September 2021, the National Institutes of Health (NIH) announced the renewal of three awards amounting to $73.2 million over five years to support the development of the Clinical Genome (ClinGen) resource. ClinGen is an initiative focused on gathering and storing data on clinically significant genes and genomic variants to facilitate precision medicine applications.
These advancements in genomic technologies have facilitated the discovery of new therapeutic targets and biomarkers for non-oncological conditions. For example, in cardiovascular diseases, genetic variations associated with cholesterol metabolism, blood clotting, and cardiac function have been identified, leading to the development of targeted therapies and personalized treatment plans.
In addition, genomic technologies have enabled the identification of rare genetic disorders and the development of tailored treatments for patients with these conditions. Through whole-exome sequencing and targeted genetic testing, clinicians can pinpoint specific genetic mutations responsible for rare diseases and design personalized interventions, including gene therapies and precision drugs.
Furthermore, the integration of genomic data with electronic health records (EHRs) and clinical databases has facilitated the discovery of genotype-phenotype correlations and the development of predictive models for disease risk assessment. By leveraging machine learning algorithms and artificial intelligence, healthcare providers can analyze large-scale genomic and clinical datasets to identify individuals at high risk of developing certain non-oncological diseases and initiate preventive measures or early interventions.
The continuous advancements in genomic technologies, such as single-cell sequencing, long-read sequencing, and epigenetic profiling, hold further promise for non-oncology precision medicine market. In January 2023, Apollo Hospitals Navi Mumbai India, a leading quaternary care hospital, introduced the Apollo Genomics Institutes. This specialized facility aims to deliver holistic care to individuals and families affected by genetic disorders. By leveraging advanced genomic technologies, the institute provides personalized and tailored medical treatments, enhancing precision medicine approaches. These technologies provide deeper insights into cellular processes, gene regulation, and disease mechanisms, allowing for more accurate diagnosis, prognosis, and treatment selection.
The Role of Growing Demand for Personalized Medicine
The growing demand for personalized medicine is a significant driver for the non-oncology precision medicine market. Patients and healthcare providers are increasingly recognizing the limitations of the traditional one-size-fits-all approach to treatment and are seeking more tailored and effective therapeutic options. Personalized medicine considers an individual’s unique genetic makeup, lifestyle factors, environmental exposures, and disease characteristics to optimize treatment outcomes. By identifying specific molecular targets and biomarkers, personalized medicine enables the development of targeted therapies and interventions that have higher efficacy and fewer adverse effects.
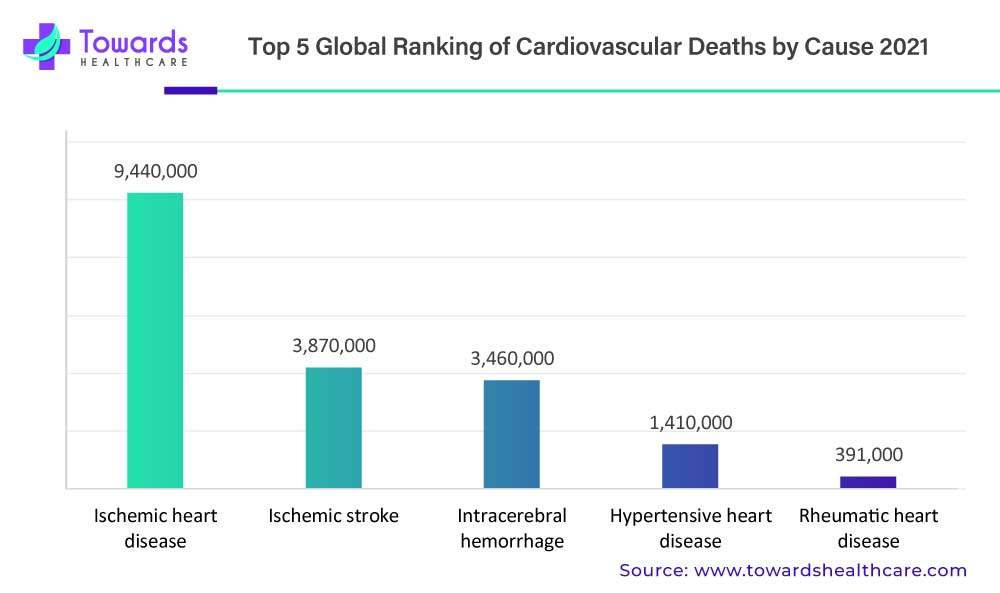
In non-oncological diseases, personalized medicine offers opportunities for early detection, prevention, and disease management. For example, in cardiovascular diseases, genetic testing can identify individuals with a higher risk of developing conditions such as hypertension or dyslipidemia. With this knowledge, healthcare providers can implement lifestyle modifications, prescribe appropriate medications, and closely monitor these individuals to prevent or delay disease progression. Cardiovascular disease claims the life of one person in the United States every 33 seconds, resulting in approximately 695,000 deaths in 2021 alone.
Unraveling the Growth Drivers: A Deep Dive
The ascent of this market is intricately linked to the surge in clinical trials and a commendable uptick in product approvals. These pivotal factors have synergized, fostering an environment conducive to robust expansion. Let’s dissect these drivers to comprehend the forces steering this remarkable journey.
In neurology, personalized medicine approaches are being employed for conditions such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Genetic testing and biomarker analysis aid in early diagnosis, risk assessment, and the development of targeted therapies that address the specific underlying molecular mechanisms of these diseases. In 2022, a total of 12 personalized medicines were identified and approved, accounting for approximately 34 percent of all newly approved drugs during that year. This indicates the growing significance and recognition of personalized medicine approaches in the pharmaceutical industry.
Furthermore, infectious diseases can benefit from personalized medicine strategies. Genetic variations in host immune response genes can influence susceptibility to infections, response to treatments, and vaccine efficacy. By identifying these genetic variations, healthcare providers can tailor treatment regimens, select appropriate antiviral or antibiotic agents, and improve patient outcomes.
The increasing availability of direct-to-consumer genetic testing and the rise of patient empowerment further contribute to the demand for personalized medicine. Patients are becoming more proactive in managing their health and seek genetic testing to gain insights into their disease risk profile and make informed decisions about their lifestyle and preventive measures.
The combination of advancements in genomic technologies and the growing demand for personalized medicine creates a conducive environment for the expansion of the non-oncology precision medicine market. Market players should focus on developing innovative diagnostic tools, biomarker discovery platforms, and targeted therapies that align with the principles of personalized medicine.
The Impact of Ethical and Legal Challenges
Ethical and legal challenges pose a major restraint for the non-oncology precision medicine market. The collection, storage, analysis, and sharing of genetic and personal health information raise concerns regarding patient privacy, data security, informed consent, and potential misuse of sensitive information. Genetic data is unique and inherently identifiable to individuals and their families, raising privacy concerns. Unauthorized access, data breaches, or misuse of genetic data can result in discrimination, stigmatization, and psychological distress for patients. Protecting patient privacy and ensuring secure storage and transmission of genetic data is critical for maintaining public trust in precision medicine.
Informed consent is another ethical consideration in the context of non-oncology precision medicine market. Patients need to fully understand the implications of genetic testing, including the potential risks, limitations, and the use of their data for research purposes. Healthcare providers must ensure that patients have access to genetic counseling and education to make informed decisions about genetic testing and the use of their genetic information.
The legal landscape surrounding genetic data and precision medicine is complex and varies across jurisdictions. Regulatory frameworks must address issues such as data ownership, data sharing, liability, and intellectual property rights. Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe, adds another layer of complexity for market players operating globally.
Moreover, the interpretation of genetic data and the determination of disease risk are not always straightforward. Genetic variations and mutations may have different implications depending on an individual’s specific genetic background and environmental factors. The complexity of genetic information requires careful communication and counseling to avoid misinterpretation or overdiagnosis.
Furthermore, there is a risk of exacerbating health disparities and inequities in access to precision medicine. Genetic testing and targeted therapies may not be equally accessible or affordable to all populations, leading to disparities in healthcare outcomes. Ensuring equitable access to precision medicine requires addressing issues of affordability, education, and healthcare infrastructure.
To overcome these ethical and legal challenges, market players in non-oncology precision medicine market should prioritize transparency, patient education, and data security. Developing clear policies and protocols for data collection, storage, and sharing, while respecting patient privacy rights, is crucial. Collaborating with regulatory bodies and stakeholders to establish ethical guidelines and standards can also foster public trust and facilitate the responsible implementation of precision medicine.
Geographical Landscape of the Non-Oncology Precision Medicine Market
North America dominates the non-oncology precision medicine market, with the United States as the largest market share holder. The region’s strong healthcare infrastructure, significant investments in precision medicine research, and presence of key market players contribute to its leadership position. The United States has witnessed a surge in public and private initiatives aimed at advancing precision medicine. The Precision Medicine Initiative launched by the U.S. government focuses on accelerating the development and adoption of precision medicine approaches across various disease areas. The initiative promotes collaborations between academia, industry, and healthcare organizations to facilitate research, data sharing, and clinical implementation of precision medicine.
The Asia-Pacific region is expected to exhibit the fastest growth rate in the non-oncology precision medicine market. Within the region, China stands out as a major contributor to this growth, driven by factors such as a large population, increasing healthcare expenditure, and government initiatives to promote precision medicine. China has witnessed rapid advancements in genomics, bioinformatics, and data analytics, positioning the country at the forefront of precision medicine research and implementation. The Chinese government has launched initiatives to support precision medicine, including the Precision Medicine Initiative and the Healthy China 2030 Plan, which aim to promote personalized healthcare and disease prevention.
Integration of Artificial Intelligence and Machine Learning
The integration of artificial intelligence (AI) and machine learning (ML) technologies presents a significant opportunity in the non-oncology precision medicine market. AI and ML have the potential to transform healthcare by analyzing large-scale genomic data, clinical records, and real-time patient monitoring to generate actionable insights and support clinical decision-making. AI and ML algorithms can identify patterns, correlations, and predictive models that aid in disease diagnosis, risk assessment, and treatment selection. By analyzing diverse datasets and integrating genetic, environmental, and lifestyle factors, AI-powered systems can provide more accurate and personalized recommendations for patients.
In non-oncology precision medicine market, AI and ML can assist in the interpretation of complex genetic data, identification of disease biomarkers, and prediction of treatment responses. These technologies can enable clinicians to stratify patient populations based on disease subtypes, genetic variations, and response profiles, leading to targeted therapies with higher efficacy.
Moreover, AI and ML algorithms can support the development of predictive models for disease prevention and early intervention. By leveraging data from wearable devices, electronic health records, and genetic profiles, AI-powered systems can identify individuals at high risk of developing non-oncological diseases and recommend preventive measures, lifestyle modifications, or personalized screening programs. The integration of AI and ML in precision medicine also holds promise for accelerating drug discovery and development.
By analyzing large datasets on disease mechanisms, drug targets, and molecular interactions, AI algorithms can identify novel therapeutic targets and facilitate the design of precision drugs. ML models can also enhance the efficiency of clinical trials by identifying patient populations most likely to respond to specific treatments, thus optimizing trial design and reducing costs.
Market players in non-oncology precision medicine market should invest in AI and ML capabilities, develop robust data infrastructure, and collaborate with technology partners to harness the power of these technologies. Data security, privacy, and regulatory compliance must be prioritized to ensure the responsible and ethical use of AI in precision medicine.
The Driving Forces: Clinical Trials and Product Approvals
Clinical Trials: Catalysts of Progress
The increasing focus on clinical trials within the realm of non-oncology precision medicine market has been a game-changer. As researchers and practitioners invest in rigorous trials, the depth of understanding and the precision of medical interventions elevate. This, in turn, propels the market forward, fueled by the promise of groundbreaking discoveries.
Product Approvals: Paving the Path to Market Maturation
The approval of new products marks a pivotal moment in the lifecycle of non-oncology precision medicine. As regulatory bodies give their nod of affirmation, the market gains momentum, offering a spectrum of innovative solutions to healthcare practitioners and patients alike. This continual influx of approved products contributes significantly to the market’s projected growth.
The Trajectory Ahead: Charting the Course for 2023-2032
Projected Expansion: A Comprehensive Outlook
Forecasts predict a compelling narrative for the non-oncology precision medicine market, with a projected Compound Annual Growth Rate (CAGR) of 8.7% from 2023 to 2032. This trajectory foresees a landscape where innovation, research, and market dynamics converge, opening avenues for unprecedented advancements.
Strategic Insights: Navigating the Competitive Terrain
As the market expands, understanding the competitive landscape becomes paramount. Companies operating in this space must harness strategic insights to not only survive but thrive. The interplay of competition and collaboration will undoubtedly shape the industry’s future.
Market Segments
By Product Type
- Diagnostics
- Genetic Tests
- Biomarker Based Tests
- Others
- Therapeutics
By Application
- Infectious Diseases
- Respiratory Infections
- Gastrointestinal Infections
- Sexually Transmitted Infections
- Others
- Neurology
- Neurodegenerative Disorders
- Neuropsychiatric Disorders
- Others
- Cardiovascular
- Cardiac Myopathies and Arrhythmia
- Others
- Lifestyle and Endocrinology
- Gastroenterology
- Others
By End-use
- Hospitals
- Diagnostic Centers
- Research & Academic Institutes
- Others
By Ecosystem
- Applied Sciences
- Genomics
By Technology
- Polymerase Chain Reaction (PCR)
- Precision Medicine Next-Generation Sequencing (PM NGS)
- Genome Editing
- Other Technologies
- Pharmacogenomics
- Other Applied Sciences
- Precision Diagnostics
- Molecular Diagnostics (MDx)
- Medical Imaging
- Digital Health and Information Technology
- Clinical Decision Support Systems (CDSS)
- Big Data Analytics
- IT Infrastructure
- Genomics Informatics
- In-Silico Informatics
- Mobile Health
- Precision Therapeutics
- Clinical Trials
- Cell Therapy
- Drug Discovery and Research
- Gene Therapy
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
Conclusion: Embracing the Future of Non-Oncology Precision Medicine
In conclusion, the non-oncology precision medicine market stands at the threshold of a transformative era. Propelled by the momentum of clinical trials, product approvals, and an unyielding commitment to innovation, its journey is one of resilience and promise. Stakeholders, from researchers to investors, are poised to witness a dynamic evolution that redefines the boundaries of healthcare possibilities. As the market forges ahead, it beckons us to embrace a future where precision medicine transcends boundaries and catalyzes a new era of healthcare excellence.