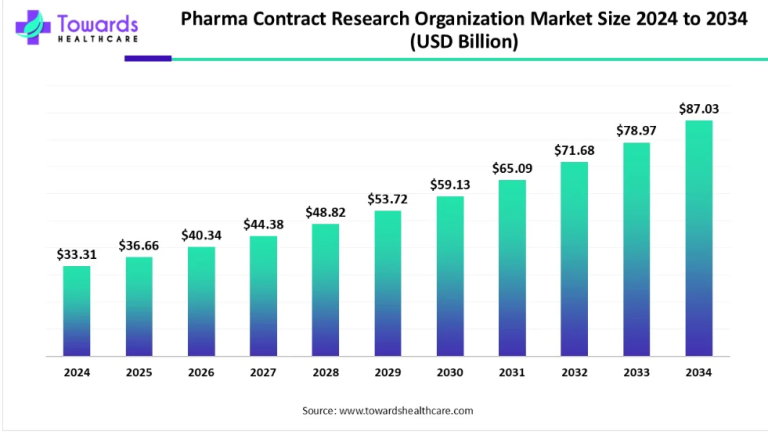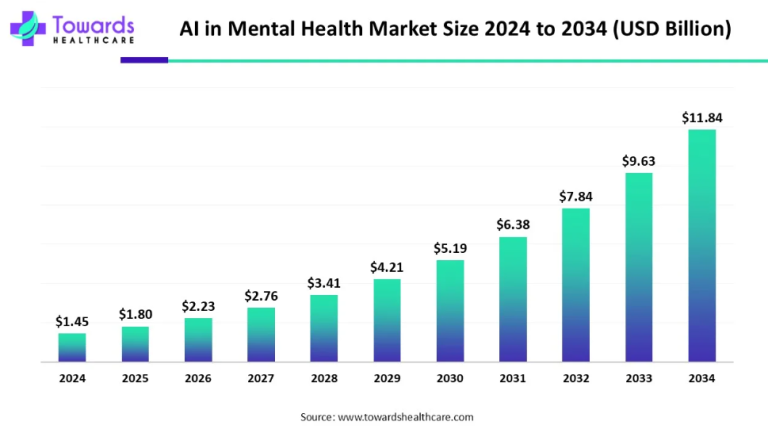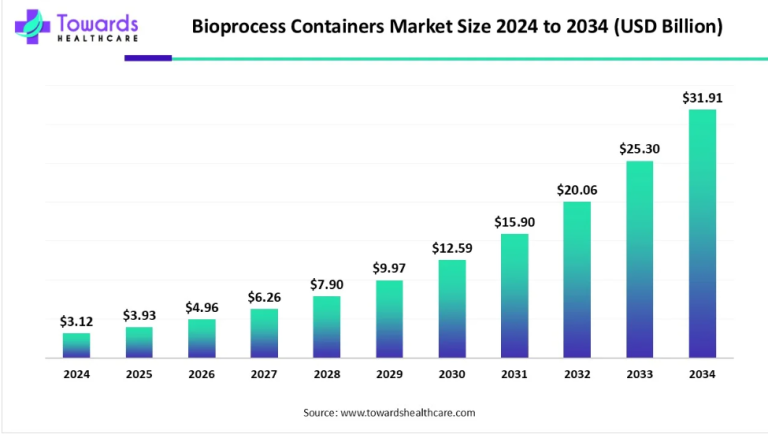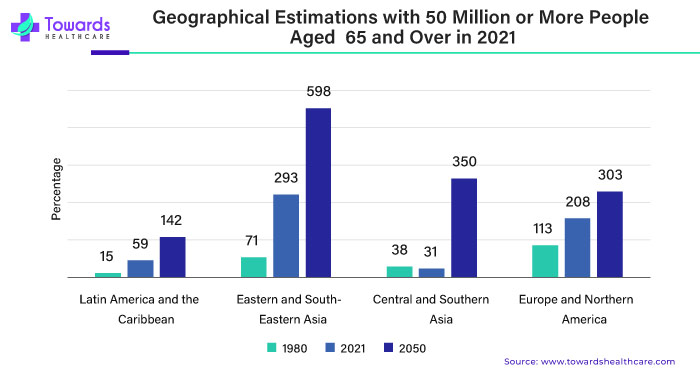
The landscape of Crohn’s disease treatment has seen significant advancements in recent years, with pharmaceutical giants like AbbVie Inc. and Pfizer Inc. making notable strides in the field. These developments are not only transforming the way we approach the management of this chronic condition but also offering hope and relief to millions of patients worldwide.
AbbVie’s Breakthrough: RINVOQ Approval
In May 2023, AbbVie Inc. achieved a significant milestone with the approval of RINVOQ (upadacitinib) by the U.S. Food and Drug Administration (FDA) for the treatment of adults with moderately to severely active Crohn’s disease. This groundbreaking approval represents a major leap forward in the realm of Crohn’s disease management, providing healthcare professionals and patients with a new and effective therapeutic option.
RINVOQ, a novel Janus kinase (JAK) inhibitor, works by targeting the underlying inflammation associated with Crohn’s disease, thereby helping to alleviate symptoms and improve patients’ quality of life. The approval of RINVOQ underscores AbbVie’s commitment to innovation and its dedication to addressing the unmet needs of patients living with inflammatory conditions.
With RINVOQ now available as a treatment option, healthcare providers have an additional tool in their arsenal to combat Crohn’s disease, offering hope for improved outcomes and better disease management for patients.
For any queries, feel free to reach us @ https://www.towardshealthcare.com/personalized-scope/5132
Pfizer’s Strategic Acquisition: Arena Pharmaceuticals
In March 2022, Pfizer Inc. made a strategic move to bolster its portfolio in the field of gastrointestinal health and inflammation by acquiring Arena Pharmaceuticals. This acquisition not only expands Pfizer’s range of products but also strengthens its expertise in areas such as stomach and bowel health, skin conditions, and heart health.
One of the key benefits of the acquisition is the access to Arena Pharmaceuticals’ innovative pipeline of products, including potential therapies for inflammatory bowel diseases like Crohn’s disease. By integrating Arena Pharmaceuticals’ research and development capabilities into its own infrastructure, Pfizer aims to accelerate the development of new treatments and bring them to market more efficiently.
Furthermore, the acquisition of Arena Pharmaceuticals allows Pfizer to leverage its resources and global reach to maximize the impact of these novel therapies, ultimately benefiting patients worldwide.
Impact on Patient Care and Beyond
The recent developments in Crohn’s disease treatment have far-reaching implications for patients, healthcare providers, and the healthcare industry as a whole. With the approval of RINVOQ and Pfizer’s strategic acquisition of Arena Pharmaceuticals, there is renewed optimism surrounding the management of this complex condition.
Patients living with Crohn’s disease now have access to a wider range of treatment options, tailored to their individual needs and preferences. This not only improves their quality of life but also empowers them to take an active role in managing their health.
Healthcare providers, armed with the latest advancements in therapy, are better equipped to provide comprehensive care and support to their patients. By staying abreast of the latest research and treatment options, they can optimize patient outcomes and ensure the best possible care for those living with Crohn’s disease.
Furthermore, the collaboration between pharmaceutical companies and healthcare providers fosters innovation and drives progress in the field of inflammatory bowel diseases. By working together to develop new therapies and improve existing treatments, we can continue to advance our understanding and management of Crohn’s disease, ultimately improving the lives of patients worldwide.
Understanding the Role of Oral NSAIDs in Pain Management
Oral NSAIDs stand as stalwarts in the realm of pain relief and inflammation control. Their diverse forms, ranging from tablets to liquids, offer a convenient solution for individuals grappling with conditions like arthritis, headaches, menstrual cramps, and injuries. This accessibility, whether over-the-counter or via prescription, ensures prompt relief for those in need.
The Versatility of Oral NSAIDs
Unlike their counterparts targeting localized pain, oral NSAIDs operate systemically, offering widespread alleviation across the body. This versatility makes them the preferred choice for many patients dealing with a spectrum of pain and inflammation-related issues. Safety and efficacy reign supreme in driving their demand, supported by extensive research confirming their effectiveness in managing moderate to severe discomfort.
Advantages Driving Patient Compliance
The ease of administration associated with oral NSAIDs fosters patient adherence to treatment plans, crucial for chronic conditions requiring sustained pain management. Moreover, with improving healthcare infrastructure globally, there’s a burgeoning demand for accessible and cost-effective pain relief options, further fueling the popularity of oral NSAIDs.
Evolution in Formulations: Meeting Market Demands
Continuous innovation in oral NSAID formulations, including extended-release variants, underscores their adaptability to evolving market needs. With an aging population and a surge in chronic pain cases, these advancements ensure oral NSAIDs remain indispensable in the pain management landscape.
Rising Preference for Alternative Therapies: A Competitive Landscape
Exploring Alternative Pain Management Strategies
Alternative therapies such as physical therapy, acupuncture, and herbal supplements are gaining traction as people seek holistic approaches to pain management. These modalities, emphasizing natural remedies and holistic healing, present viable alternatives to traditional medications like NSAIDs.
Challenges to NSAIDs: Navigating the Competitive Terrain
The burgeoning popularity of alternative therapies poses a challenge to NSAIDs, necessitating pharmaceutical companies to differentiate and highlight the unique advantages of their products. While alternative therapies boast reduced side effects and holistic wellness benefits, NSAIDs offer quick, targeted relief backed by robust clinical evidence.
Navigating Market Dynamics: Ensuring Continued Relevance
To maintain their competitive edge, pharmaceutical firms must invest in research and development, focusing on enhancing NSAID formulations. By showcasing the rapid onset of action and predictable dosing of NSAIDs, companies can reaffirm their significance in the pain management market.
Embracing Collaboration for Optimal Patient Care
A collaborative approach, bridging conventional and alternative medicine, offers patients a comprehensive pain management strategy. By synergizing the benefits of NSAIDs with alternative therapies, healthcare providers can tailor solutions that meet the diverse needs of patients, ensuring optimal outcomes.
North America: A Hub for Non-Steroidal Anti-Inflammatory Drugs
In North America, the demand for Non-steroidal Anti-inflammatory Drugs (NSAIDs) is substantial, driven by the prevalence of health issues like arthritis and chronic pain among its large population. With advanced healthcare infrastructure, accessing these medications is relatively easy. Renowned companies invest heavily in research to ensure the safety and efficacy of their NSAIDs, alongside robust marketing efforts to raise awareness. Regulatory bodies such as the FDA and Health Canada rigorously evaluate and monitor these drugs, ensuring compliance with safety standards throughout their lifecycle.
Asia Pacific: Rising Demand Amidst Growing Healthcare Infrastructure
The Asia Pacific region witnesses a surge in demand for NSAIDs due to its vast population and increasing incidence of chronic diseases like arthritis. Government policies significantly influence the NSAIDs market dynamics, particularly regarding pricing, reimbursement, and approval processes. Rapid economic growth in Southeast Asia and South Asia contributes to higher affordability, expanding the consumer base for these medications.
Innovations and Market Expansion
In May 2021, Ono Pharmaceutical Co., Ltd. introduced JOYCLU 30mg injection for joint-related ailments in Japan, following regulatory approval. This milestone enhances accessibility to NSAIDs, presenting lucrative opportunities for pharmaceutical companies. The competitive landscape in the Asia Pacific necessitates continual product enhancements and innovative delivery mechanisms to thrive in this evolving market.
Unlock Infinite Advantages: Subscribe to Annual Membership
To own our Premium Research Study Instantly, Click here: https://www.towardshealthcare.com/price/5132
Read more: