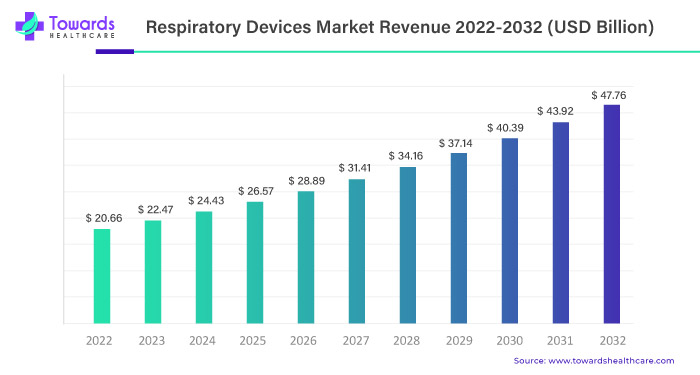
In the dynamic landscape of healthcare, the respiratory devices market is poised for a remarkable ascent, projecting a robust growth trajectory. Anticipated to surge from USD 20.66 billion in 2022, the market is set to ride an impressive 8.74% Compound Annual Growth Rate (CAGR) between 2023 and 2032. This growth surge is foreseen to culminate in an estimated market value of USD 47.76 billion by the year 2032.
 Let’s delve into the driving forces behind this phenomenal surge, propelled chiefly by the escalating prevalence of respiratory disorders.
Let’s delve into the driving forces behind this phenomenal surge, propelled chiefly by the escalating prevalence of respiratory disorders.
Breathing Life into Market Dynamics: Unraveling the Respiratory Devices Market
1. Pervasive Respiratory Disorders Ignite Market Momentum
The spark behind the market’s meteoric rise lies in the escalating prevalence of respiratory disorders. As the global population grapples with an increasing burden of respiratory ailments, the demand for advanced and efficient respiratory devices market are skyrocketing. From chronic obstructive pulmonary disease (COPD) to asthma, these conditions are propelling the adoption of cutting-edge respiratory solutions.
2. Technological Innovations Redefining the Landscape
In the quest for optimal patient outcomes, technological advancements are reshaping the respiratory devices market landscape. Innovations such as smart inhalers, portable ventilators, and advanced nebulizers are not only enhancing treatment efficacy but also fostering patient convenience. This paradigm shift towards technologically sophisticated solutions is playing a pivotal role in bolstering market expansion.
3. Rising Healthcare Awareness: A Catalyst for Market Growth
A global surge in healthcare awareness is contributing significantly to the burgeoning demand for respiratory devices market. With an increasing emphasis on preventive healthcare, individuals are becoming more proactive in managing respiratory health. This proactive stance is translating into a higher adoption rate of respiratory devices market, driving growth to unprecedented levels.
US FDA Medical Device Approvals
During the pandemic crisis, regulators experienced difficulty in coping with added obligations, and acceptable device approval procedures slowed. In line with this, data from H1 2021 show a substantial decline in premarket authorizations (PMAs) (12) and 510(k) approvals (1,360) compared to H1 2020.
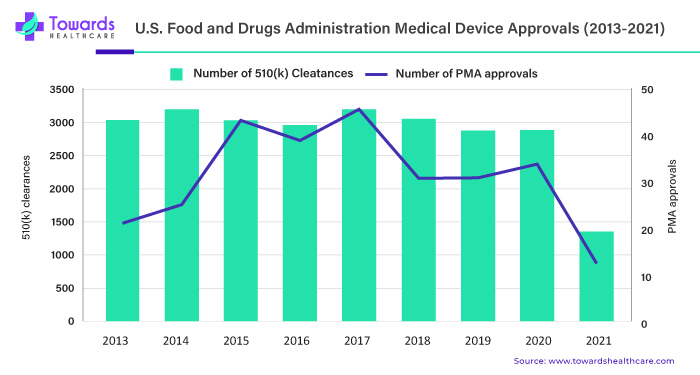
By adopting the FDA’s recommended Total Product Lifecycle (TPLC) approach, one of the sector’s legislative objectives should be to render quicker launches a long-term reality. According to an FDA paper published in February 2021, “the FDA’s traditional paradigm of medical device regulation was not designed for adaptive artificial intelligence and machine learning technologies.”
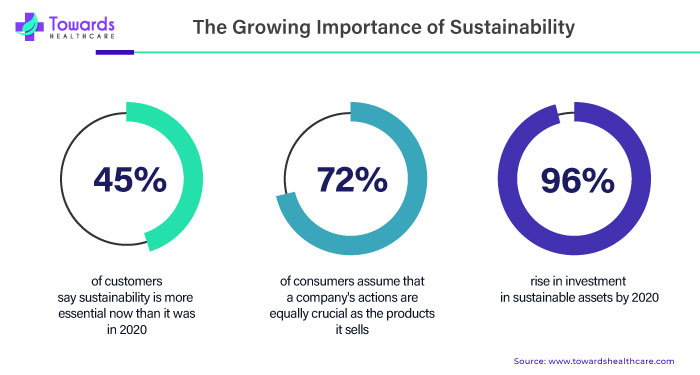
The Growing Importance of Sustainability in MedTech:
Sustainability should be a top priority for MedTech. According to a 2020 Health Affairs analysis, the global health sectors produced 4.6% of all greenhouse emissions (double that of the aviation industry), using medical device supply chains demonstrating significant opportunities to foster more sustainable practices. (In 2018, alone, device reprocessing reduced hospital waste generation by 7,100 tonnes in the United States, Europe, and Canada.)
Several leading medical technology companies have adopted sustainability practices:
- In February 201, Phillips accomplished carbon neutrality throughout its businesses, with nearly all of its electricity generated sustainably, 90% of its waste from operations recycled, and 15% of its revenue coming from circular revenues.
- In November 2020, Medtronic, Boston Scientific, Illumina, Edwards, and Becton Dickinson became part of the S&P Dow Jones Sustainability North America Index. As a result of its expansive Health for Humanity sustainability initiative, Johnson & Johnson gathered around 1.6 million medical devices and reprocessed 670,000 in 2020.
As devices encompass more digital and electronic items, waste issues expand beyond single-use plastics and evolve into increasingly serious ones. From production methods to packaging and product recycling in the the final year of their shelf life, there are numerous fields where MedTechs can concentrate on lowering not only CO2 but also the wider environmental impacts of their goods.
The Role of Growing Number of MedTech Mergers and Acquisitions
MedTech companies completed 288 M&A transactions in the fiscal year ending June 2021, the most in a single year since 2007.
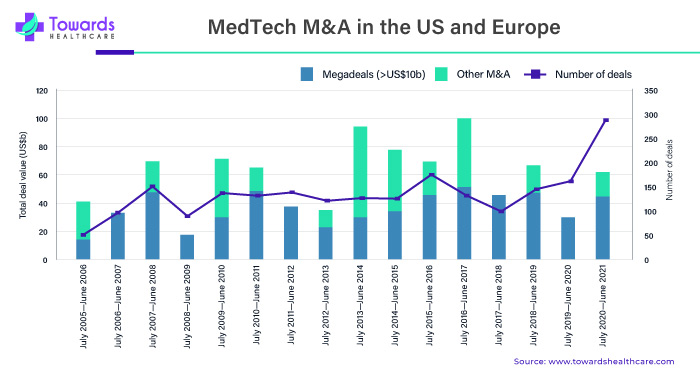
“MedTechs are making substantial investments in distant medical capacities. Baxter agreed to buy connected care specialist Hillrom for $10.5 billion in September 2021. Patients constantly desire to get medical treatment at their residence or nearby,” CEO Jose (Joe) Almeida told Ernst & Young LLP, “whereas medical facilities and other healthcare facilities are turning to digital health tools to broaden accessibility, enhance effectiveness, and reduce costs.” Baxter and Hillrom have joined forces to address the challenges of a constantly shifting global healthcare landscape.
The Growing Importance of Sustainability in MedTech:
Sustainability should be a top priority for MedTech. According to a 2020 Health Affairs analysis, the global health sectors produced 4.6% of all greenhouse emissions (double that of the aviation industry), using medical device supply chains demonstrating significant opportunities to foster more sustainable practices. (In 2018, alone, device reprocessing reduced hospital waste generation by 7,100 tonnes in the United States, Europe, and Canada.)
Several leading medical technology companies have adopted sustainability practices:
- In February 201, Phillips accomplished carbon neutrality throughout its businesses, with nearly all of its electricity generated sustainably, 90% of its waste from operations recycled, and 15% of its revenue coming from circular revenues.
- In November 2020, Medtronic, Boston Scientific, Illumina, Edwards, and Becton Dickinson became part of the S&P Dow Jones Sustainability North America Index. As a result of its expansive Health for Humanity sustainability initiative, Johnson & Johnson gathered around 1.6 million medical devices and reprocessed 670,000 in 2020.
As devices encompass more digital and electronic items, waste issues expand beyond single-use plastics and evolve into increasingly serious ones. From production methods to packaging and product recycling in the the final year of their shelf life, there are numerous fields where MedTechs can concentrate on lowering not only CO2 but also the wider environmental impacts of their goods.
Respiratory Disorders are on the Rise
Rising cases of respiratory disorders such as respiratory cancers, asthma, COPD, tuberculosis, and others across the globe are prominently generating demands for respiratory devices market, which ultimately results in the respiratory devices market growth. Here are some of the facts related to the respiratory disorders:
- Around 7.7% US population which is equal to around 25 million people was detected with Asthma in 2021, which was reported to increase from 7.4% or 20.3 million US population in 2001.
- As stated by the Global Asthma Report 1 in 10 children across the globe have asthma symptoms, many of which have no access to vital asthma treatment.
- In the UK, respiratory conditions are the 3rd largest cause of death and one person gets diagnosed with a respiratory condition every minute.
- COPD affects one in every ten adults worldwide and is one of the three leading causes of death. COPD killed 3.22 million people in 2019. COPD is the leading cause of death in Latin America, Sub-Saharan Africa, India, China, and Southeast Asia.
In addition, rising tobacco smoking, air pollution, and allergens are some of the major risk factors that are resulting in respiratory disease conditions. Tobacco smoking is considered one of the world’s largest health problems. In 2021, around 28.3 million US adults (approximately 13.1% of males and 10.1% of females) smoked cigarettes. In 2020, 22.3% of the global population used tobacco: 36.7% of males and 7.8% of females. Thus, rising tobacco consumption and air pollution result in severe respiratory health conditions and increases the demand for the respiratory device, which in turn bolsters the respiratory devices market growth.
High Purchase Prices and Maintenance Costs
The high cost associated with advanced respiratory devices market such as oxygen concentrators, ventilators, continuous positive airway pressure (CPAP) machines, and several others acts as a barrier to the growth of the respiratory devices market. As a result of the high cost of product, it limits the extensive adoption of such devices especially in medical facilities with low resources.
In cases of severe respiratory distress, including in patients undergoing surgeries requiring general anesthesia or patients with acute respiratory distress syndrome (ARDS) ventilators are essential life-supporting tools. These exhaustive devices can be highly expensive. On top of it, for proper operation and patient safety, ventilators need routine maintenance, calibration, and servicing. The price of maintenance agreements, replacement parts, and certified technicians also increases the overall cost of the device, which in turn hinders market growth. During the COVID-19 pandemic, there was a massive increase in the demand for ventilators.
As a result, the cost of mechanical ventilators—basic but effective equipment—rose sharply, from an average of $25,000 to more than $50,000. Several countries with limited healthcare facilities and low medical budgets suffered from an insufficient number of ventilators due to their high costs. In addition, Obstructive sleep apnea is a breathing disorder that is frequently treated with continuous positive airway pressure (CPAP) machines. CPAP machines can be expensive, especially for patients without medical coverage, despite being less expensive than ventilators. The price includes the CPAP machine itself, as well as filters, masks, and, hoses. CPAP machines also require routine maintenance and eventually need to be replaced, which further increases the overall cost of the device.
Forecasting Tomorrow: The Trajectory Ahead
As we navigate through the evolving landscape of respiratory devices market healthcare, the market’s trajectory is poised for an upward spiral. With technological innovations, a surge in healthcare awareness, and the persistently rising prevalence of respiratory disorders, the respiratory devices market is set to be a focal point of growth and innovation. Stakeholders in this domain must align their strategies with these transformative trends to harness the full potential of this burgeoning market.
Recent Developments:
- In April 2023, Masimo received FDA approval for a monitoring set of pulse oximeters, respiration rate from the pleth, named Rad-G with Temperature. Rad-G with Temperature makes it simpler for care teams to quickly assess patients anywhere patient assessment is required by using a single, portable, compact device to measure vital signs.
- In May 2021, Medtronic a medical device manufacturer launched a U.S. commercial pediatric airway monitoring system called SonarMed. Using acoustic technology, the system checks for endotracheal tube (ETT) obstruction and examines position in real-time, providing doctors with the essential data they need to make smarter, life-saving choices for their smallest patients.
- In March 2020, GE Healthcare partnered with Ford in order to fast-track ventilator manufacturing to support doctors in the treatment of COVID-19 patients.