Gene therapy has emerged as a promising frontier in the treatment of various genetic disorders, offering hope where traditional pharmaceutical interventions fall short. One such recent development comes from 4D Molecular Therapeutics, a biotechnology company at the forefront of innovation in genetic medicine.
Introduction to X-linked Retinitis Pigmentosa
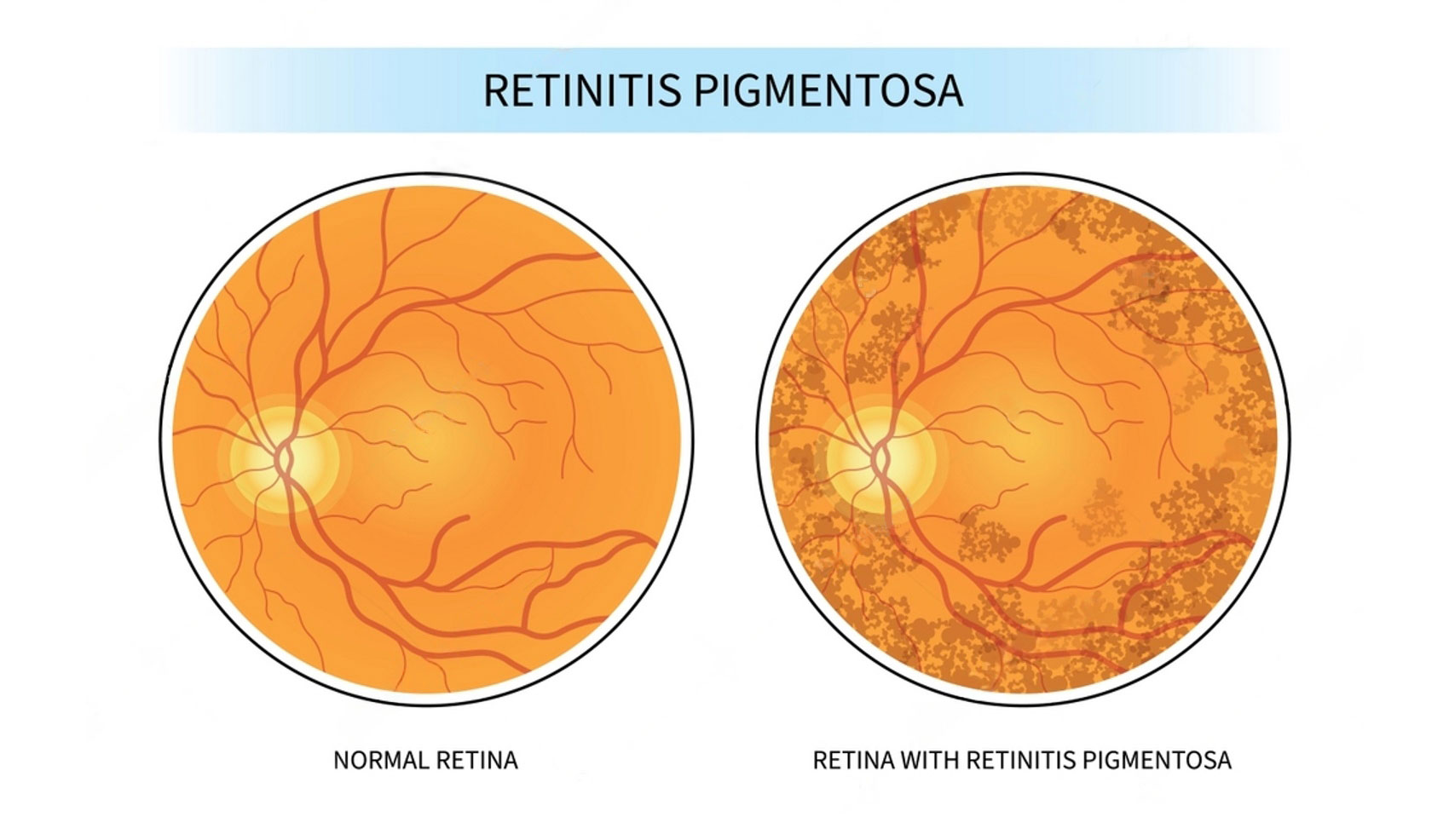
Before delving into the breakthrough treatment, it’s crucial to understand the condition it aims to address. X-linked retinitis pigmentosa (XLRP) is a rare inherited retinal disorder characterized by progressive vision loss. This condition primarily affects males and often manifests in childhood or adolescence, gradually worsening over time. The underlying cause of XLRP lies in mutations of the RPGR gene, essential for maintaining the health of photoreceptor cells in the retina.
The Significance of 4D-125
In January 2022, 4D Molecular Therapeutics received notable recognition from the U.S. Food and Drug Administration (FDA) for its investigational therapy, 4D-125, designed specifically for XLRP. This groundbreaking treatment targets the root cause of the disease by delivering a functional version of the RPGR gene directly to the retinal cells. Unlike traditional treatments that merely manage symptoms, 4D-125 aims to restore vision by addressing the underlying genetic defect.
Mechanism of Action
4D-125 utilizes a sophisticated delivery system to transport the therapeutic gene to its target site within the eye. By leveraging adeno-associated viral (AAV) vectors, researchers can effectively penetrate the retinal tissue and deliver the genetic payload to the affected cells. Once inside, the therapeutic gene integrates into the host genome, enabling the production of functional RPGR protein. This protein plays a crucial role in maintaining the structural integrity of photoreceptor cells, thereby halting the progression of vision loss in individuals with XLRP.
For any queries, feel free to reach us @ https://www.towardshealthcare.com/personalized-scope/5114
Advantages of 4D-125
Precision Targeting
Unlike systemic treatments that may have off-target effects, 4D-125 offers precise targeting of the affected retinal cells. By delivering the therapeutic gene directly to the site of pathology, this approach minimizes potential side effects and maximizes therapeutic efficacy.
Minimally Invasive Administration
The design of 4D-125 allows for minimally invasive administration, making it more accessible and convenient for patients. By employing non-surgical delivery methods, such as intravitreal injection, clinicians can administer the treatment safely and efficiently in an outpatient setting.
Potential for Long-lasting Effects
One of the most promising aspects of 4D-125 is its potential for long-lasting effects. By correcting the underlying genetic defect, this therapy has the potential to provide sustained benefits, potentially halting or even reversing the progression of XLRP-related vision loss.
Future Implications and Clinical Trials
The FDA’s recognition of 4D-125 as a breakthrough therapy underscores the significance of this innovation in the field of genetic medicine. As 4D Molecular Therapeutics advances through clinical trials, the potential impact of 4D-125 on individuals with XLRP continues to garner attention from researchers, clinicians, and patients alike. Future studies will aim to further elucidate the safety, efficacy, and long-term outcomes of this promising treatment.
The FDA’s special status designation for 4D-125 represents a significant milestone in the pursuit of effective treatments for X-linked retinitis pigmentosa. By harnessing the power of gene therapy, 4D Molecular Therapeutics offers renewed hope for individuals grappling with this devastating genetic disorder. As research progresses and clinical trials unfold, the potential of 4D-125 to transform the lives of patients with XLRP remains a beacon of optimism in the realm of genetic medicine.
Unlock Infinite Advantages: Subscribe to Annual Membership
To own our research study instantly, Click here @ https://www.towardshealthcare.com/price/5114
Read More about Retinitis Pigmentosa Market: