The North American region, especially the United States, stands as a pivotal center for the development and commercialization of RNAi therapeutics. This article delves into the geographical landscape of the RNAi therapeutics market, highlighting key factors contributing to its growth and significance.
RNAi Therapeutics Development in North America
Robust Biotechnology Sector
North America boasts a robust biotechnology sector, fostering innovation and advancement in RNA interference (RNAi) technology. Companies and academic institutions across the region are actively engaged in research and development endeavors aimed at harnessing the potential of RNAi-based treatments for various diseases.
Supportive Regulatory Environment
One of the critical drivers propelling the RNAi therapeutics market in North America is the supportive regulatory environment, exemplified by agencies like the Food and Drug Administration (FDA). The FDA plays a crucial role in ensuring the safety and efficacy of RNAi-based therapies, facilitating their timely approval and commercialization.
Key Players and Research Infrastructure
The presence of key players, including biopharmaceutical companies and academic research centers, underscores North America’s prominence in RNAi therapeutics. These entities leverage state-of-the-art research infrastructure and cutting-edge technologies to accelerate the development and translation of RNAi-based interventions from bench to bedside.
For any questions, we are available for you @ https://www.towardshealthcare.com/personalized-scope/5115
Market Dynamics and Opportunities
Adoption of Innovative Therapies
North America’s well-established healthcare systems and high healthcare expenditure levels create a conducive environment for the adoption of innovative therapies, including RNAi therapeutics. Patients and healthcare providers alike benefit from access to advanced treatment modalities that offer promising outcomes and improved quality of life.
Addressing Unmet Medical Needs
RNAi therapeutics hold immense potential in addressing unmet medical needs across a spectrum of diseases, ranging from cancer to viral infections and genetic disorders. The targeted and specific nature of RNAi-based interventions offers a novel approach to disease management, complementing existing treatment modalities and filling gaps in patient care.
Comparative Analysis: Global Landscape
Asia-Pacific Region
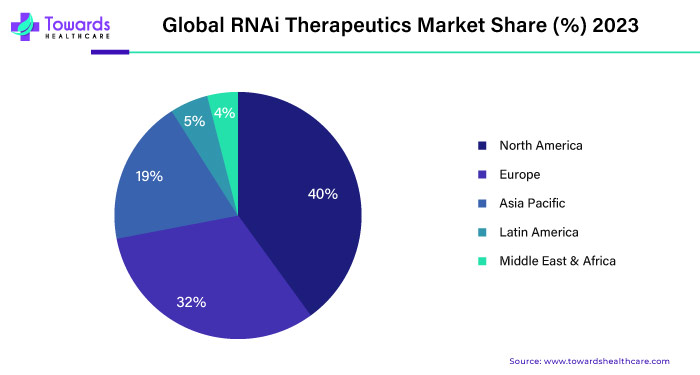
While North America leads in RNAi therapeutics development, the Asia-Pacific region presents significant opportunities for market expansion. Countries such as China, Japan, South Korea, and India are witnessing rapid growth in their biopharmaceutical industries, fueled by increasing healthcare expenditure and a growing burden of chronic diseases.
Regulatory Frameworks and Innovation
Regulatory frameworks governing RNAi therapeutics are evolving in the Asia-Pacific region, with regulatory authorities working to streamline approval processes and foster innovation. This proactive approach facilitates the translation of research findings into clinical applications, driving the growth of the RNAi therapeutics market in the region.
North America serves as a cornerstone of RNAi therapeutics development, driven by a combination of factors such as a robust biotechnology sector, supportive regulatory environment, and significant investments in research and development. While opportunities for market expansion exist globally, the region’s leadership in advancing RNAi-based interventions underscores its pivotal role in shaping the future of healthcare.
To own our study instantly, click here @ https://www.towardshealthcare.com/price/5115
Access our Premium Real Time Data Intelligence Tool, Visit: www.precedencestatistics.com
Read More Snapshots Related to RNAi Therapeutics Sector: