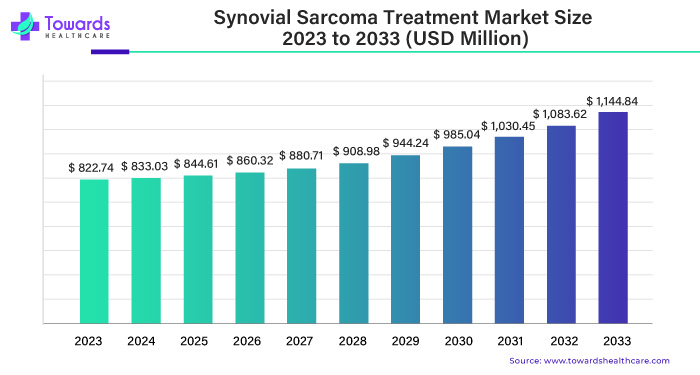
The synovial sarcoma treatment market was valued at USD 822.74 million in 2023 and is projected to grow to USD 1,144.84 million by 2033, with an estimated CAGR of 3.6% over the forecast period. Surgery continues to be the primary treatment method, commanding a substantial 56% market share in 2023. North America leads the market, accounting for 41% of the share in the same year.
Download statistics of this report @ https://www.towardshealthcare.com/download-statistics/5121
According to the National Institute of Health, synovial sarcoma, a type of mesenchymal tumor commonly diagnosed in individuals under the age of 30, requires appropriate treatment for management. As the occurrence of synovial sarcoma rises, there is an increasing demand for innovative therapies to address this medical need.
Synovial sarcoma is a type of cancer that typically affects young adults and teenagers. It starts in the soft tissues, like muscles or tendons, usually around the joints in the arms or legs. Despite its name, it doesn’t develop from the synovium, which is the lining of joints. Instead, it gets its name because, under the microscope, it looks similar to cells found in the synovium. Synovial sarcoma can be aggressive and may spread to other parts of the body. It’s often treated with a combination of surgery, chemotherapy, and radiation therapy. Early detection and treatment are essential for better outcomes.
Report From National Institute of Health
- As per the National Institute of Health, the occurrence of synovial sarcoma, a type of mesenchymal tumor commonly diagnosed in individuals under the age of 30, is on the rise with each passing day. Despite being infrequent, with an annual reporting of 800 to 1000 cases, synovial sarcoma is distinctively identified by a specific chromosomal translocation involving chromosomes X and 18. This translocation event triggers the formation of SS18:SSX fusion proteins, which are integral to the pathology of this malignancy. Consequently, the medical community is increasingly attentive to this condition due to its emerging prevalence and the molecular intricacies underlying its development.
Synovial sarcoma is crucial to treat and diagnose early because early detection and treatment can significantly improve outcomes. This type of cancer can grow and spread rapidly if left unchecked, making it harder to treat as it progresses. By catching synovial sarcoma early, doctors can intervene before it spreads to other body parts, making treatment more effective and increasing the chances of successful recovery. Additionally, early diagnosis allows for less invasive treatment options, reducing the potential for complications and improving the quality of life for patients. Therefore, it’s essential to seek medical attention promptly if there are any signs or symptoms suggestive of synovial sarcoma to ensure the best possible outcome.
For instance,
- In June 2022, Advenchen Laboratories, LLC announced that they, along with Jiangsu Chia Tai Tianqing Pharmaceutical, are starting the Phase 3 clinical trial to test a potential treatment for synovial sarcoma. The therapy, called Catequentinib (previously known as anlotinib), is a pill that blocks specific proteins in the body to help fight cancer. This trial is important in testing whether this medication is safe and effective for treating synovial sarcoma.
The market for synovial sarcoma treatment is expected to increase because more cases of this type of cancer are being diagnosed. As our understanding of the disease improves, doctors can better identify and treat it. Additionally, advances in medical research and technology are leading to the development of more effective treatments. As a result, a growing demand for medications, therapies, and medical services related to synovial sarcoma drives the market’s growth.
Increasing Prevalence of Synovial Sarcoma
The American Cancer Society projects that in 2024, approximately 13,590 new cases of soft tissue sarcomas will be diagnosed in the United States. Among these cases, around 7,700 are expected to occur in males and 5,890 in females. Additionally, it is estimated that about 5,200 individuals will succumb to soft tissue sarcomas, with approximately 2,760 males and 2,440 females losing their lives to this type of cancer.
The increasing number of synovial sarcoma diagnoses creates a pressing demand for effective treatments. Imagine a scenario where a popular toy suddenly becomes highly sought after – to meet the market, and stores ramp up production.
- In 2023, around 13,400 individuals in the United States are expected to receive a diagnosis of soft-tissue sarcoma, with about 7,400 cases in males and boys and 6,000 cases in females and girls. Additionally, it is anticipated that approximately 5,140 people will pass away from this illness in the United States during the same year, with roughly 2,720 deaths in males and boys and 2,420 deaths in females and girls.
Synovial sarcoma, the growing patient population, drives the need for more treatment options. This spurs doctors and scientists into action, prompting intensified efforts to develop innovative medicines and therapies personalized to combat the cancer’s specific characteristics. With more patients seeking treatment, pharmaceutical companies and hospitals recognize the market potential and invest heavily in research and development.
For instance,
- In March 2022, C4 Therapeutics revealed that the FDA granted Orphan Drug Designation (ODD) to CFT8634 for treating Soft Tissue Sarcoma. This medication is designed to specifically target tumors dependent on BRD9, like synovial sarcoma, and tumors with SMARCB1 deletion. C4 Therapeutics focuses on developing innovative small-molecule drugs to transform disease treatment.
This influx of resources fuels advancements in understanding the disease and creating new treatment modalities. As a result, the cycle continues: more diagnoses lead to more significant investment in research, yielding more treatment options. Ultimately, this iterative process drives the growth of the synovial sarcoma treatment market, ensuring patients have improved access to the care they urgently need.
Ongoing Research and Development
Researchers are deeply invested in unraveling the complexities of synovial sarcoma, meticulously examining its intricate components to understand how the disease operates at its core. This profound understanding is the foundation for developing targeted treatments, akin to crafting a precisely fitting key to unlock a door, where these therapies are designed to attack cancer cells while sparing healthy tissue selectively.
Additionally, the advent of personalized medicine allows for the customization of treatments to suit the individual characteristics of each patient, resembling customized-made personalized clothing to fit perfectly. Concurrently, ongoing research efforts drive the development of novel drugs customized specifically to combat synovial sarcoma, broadening the spectrum of available treatment options and potentially enhancing patient outcomes and quality of life.
For instance,
- In July 2020, the European Medicines Agency (EMA) granted special status to Adaptimmune’s ADP-A2M4 for treating synovial sarcoma. This decision gives the company extra help from experts and regulators to speed up the drug’s development, possibly making it available to patients sooner.
Furthermore, specific treatments directly assail cancer cells and bolster the body’s inherent defense mechanisms against the disease, offering an additional layer of protection. Clinical trials play a pivotal role in evaluating the safety and efficacy of these emerging treatments, providing invaluable data to refine and further develop therapeutic strategies while attracting essential funding for continued research endeavors. This relentless pursuit of understanding and innovation in synovial sarcoma treatment holds immense promise for improving patient outcomes and propelling advancements in the field.
For instance,
- In January 2022, Salarius Pharmaceuticals, Inc., a company that works on developing new medicines for various cancers, including sarcomas and pediatric cancers, made an official agreement to buy a collection of potential cancer-fighting drugs from DeuteRx, LLC. Salarius renamed the leading drug candidate SP-3164 (previously known as DRX-164) and also acquired related patents. This deal also allows Salarius to find more cancer-fighting drugs in the same area of research.
Chemotherapy is Highly Recommended for the Management of Synovial Sarcoma
Chemotherapy is essential for treating synovial sarcoma, and it helps the market for these treatments in a few ways. First, it’s often the primary treatment doctors use, especially when surgery or radiation isn’t an option. So, there’s always a need for chemotherapy drugs, which keeps the market going strong.
Additionally, chemotherapy is often used alongside other treatments like surgery or radiation to lower the chances of the cancer coming back. This makes chemotherapy a crucial part of overall treatment plans. When synovial sarcoma has spread or can’t be cured, chemotherapy is used to help manage symptoms and make patients feel better. This means there’s always a demand for these drugs, even if they can’t cure the cancer.
Combining different types of treatments for cancer is showing promise in trials
- Chemotherapy + Immunotherapy: Mixing traditional chemotherapy with drugs that boost the immune system, like pembrolizumab or nivolumab, to help the body fight cancer cells.
- Chemotherapy + Target Therapies: Pair chemotherapy with drugs that target specific molecular pathways in synovial sarcoma, aiming to improve the treatment’s effectiveness and possibly overcome resistance to drugs.
Because there’s a significant demand for better chemotherapy drugs, pharmaceutical companies keep working on new ones. This helps expand the market by giving patients more options for treatment. Furthermore, ongoing research studies, called clinical trials, test out new chemotherapy treatments. If these trials show positive results, it could lead to new drugs being approved and more choices for patients.
Synovial Sarcoma Treatment Market Companies
- Novartis
- Eli Lilly and Company
- Adaptimmune
- Takara Bio Inc.
- Johnson & Johnson
- F. Hoffmann-La Roche Ltd.
- Eisai Co., Ltd.
- Aadi Biosciences
- Immunocore Ltd.
- GlaxoSmithKline
Discover our detailed Table of Contents (TOC) for the Industry, providing a thorough examination of market segments, material, emerging technologies and key trends. Our TOC offers a structured analysis of market dynamics, emerging innovations, and regional dynamics to guide your strategic decisions in this rapidly evolving healthcare field – https://www.towardshealthcare.com/table-of-content/synovial-sarcoma-treatment-market-sizing
To own our research study instantly, Click here @ https://www.towardshealthcare.com/price/5121
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Explore the comprehensive statistics and insights on healthcare industry data and its associated segmentation: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare