Doctor examining IV drip
Understanding Synovial Sarcoma
Synovial sarcoma is a rare type of cancer that typically affects the soft tissues around joints. It accounts for only about 5-10% of all soft tissue sarcomas. Despite its rarity, synovial sarcoma presents significant challenges in treatment due to its aggressive nature and tendency to recur even after seemingly successful therapy. However, recent advancements in medical research offer hope for improved outcomes for patients battling this challenging disease.
Takara Bio’s Groundbreaking Research
In May 2023, Takara Bio, a renowned biotech company, unveiled promising findings from a NY-ESO-1-specific TCR gene therapy trial conducted in Japan. This groundbreaking research represents a significant step forward in the quest for more effective treatments for synovial sarcoma. The trial focused on harnessing the power of the immune system to target cancer cells specifically, offering a potentially less toxic and more targeted approach compared to traditional chemotherapy.
Any queries, feel free to reach us @ https://www.towardshealthcare.com/personalized-scope/5121
TBI-1301: A Potential Game-Changer
At the forefront of Takara Bio’s research efforts is TBI-1301, a novel drug developed for the treatment of synovial sarcoma. Building upon the success of the NY-ESO-1-specific TCR gene therapy trial, Takara Bio is poised to seek approval for TBI-1301 in Japan. If approved, TBI-1301 has the potential to revolutionize the treatment landscape for synovial sarcoma patients, offering new hope where previously there may have been limited options.
The Road to Approval
Following the promising results of the clinical trial, Takara Bio is diligently preparing to navigate the regulatory pathways for drug approval in Japan. This process involves rigorous scrutiny of the drug’s safety and efficacy profiles, as well as its manufacturing processes. Takara Bio’s commitment to upholding the highest standards of quality and compliance underscores their dedication to bringing innovative therapies to patients in need.
Ensuring Access to Innovative Therapies
In addition to seeking regulatory approval for TBI-1301, Takara Bio is proactively planning for the drug’s commercialization and distribution. Establishing a robust supply chain infrastructure is essential to ensure that TBI-1301 reaches patients in a timely and efficient manner once it receives regulatory clearance. This comprehensive approach demonstrates Takara Bio’s commitment to not only developing groundbreaking therapies but also ensuring that they are accessible to those who need them most.
Collaborative Efforts in Research
The journey towards advancing synovial sarcoma treatment is not one that Takara Bio undertakes alone. Collaboration with healthcare professionals, researchers, patient advocacy groups, and regulatory authorities is integral to the success of their endeavors. By fostering partnerships and leveraging collective expertise, Takara Bio aims to accelerate progress in the field of oncology and improve outcomes for patients worldwide.
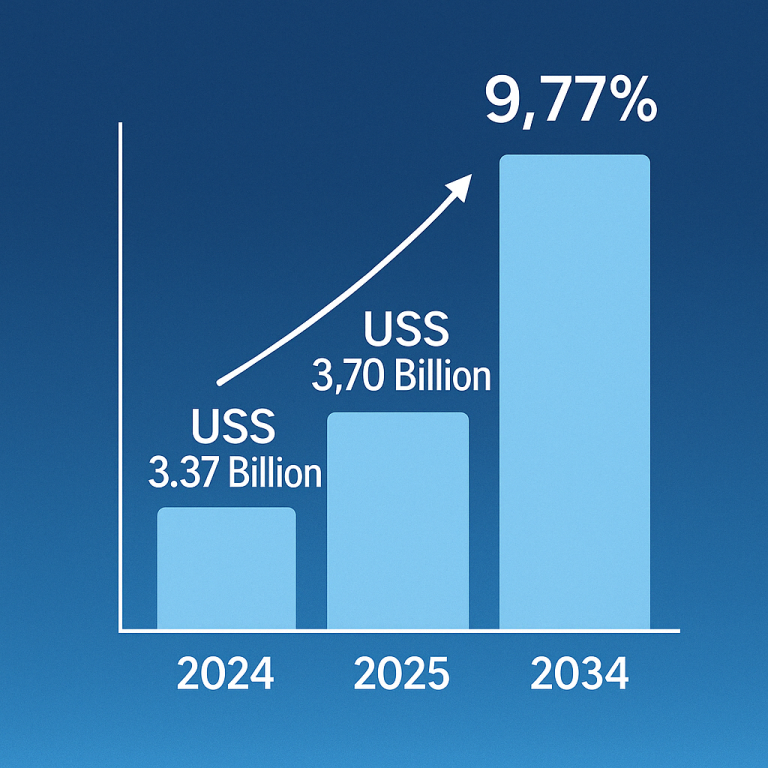
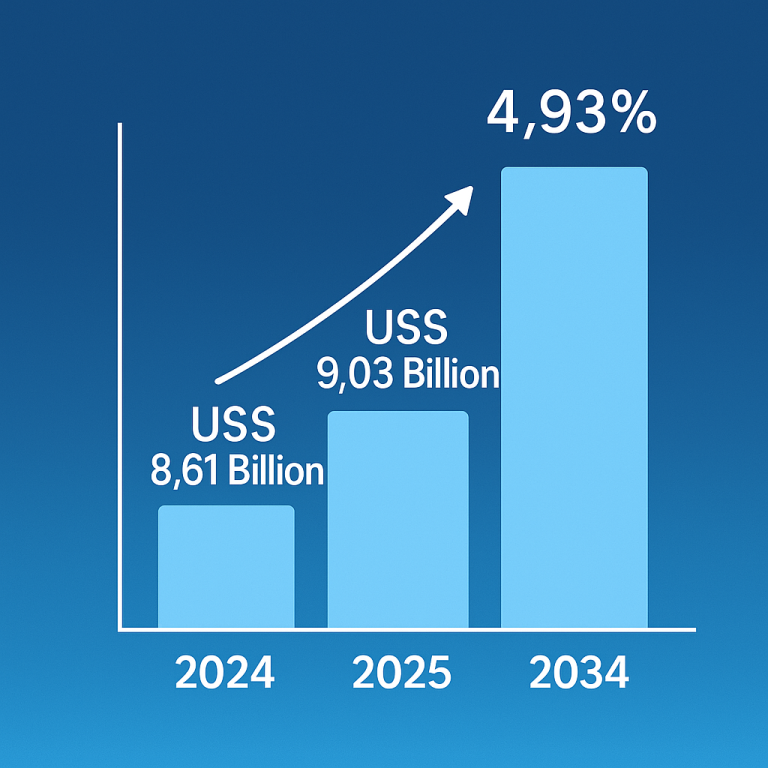
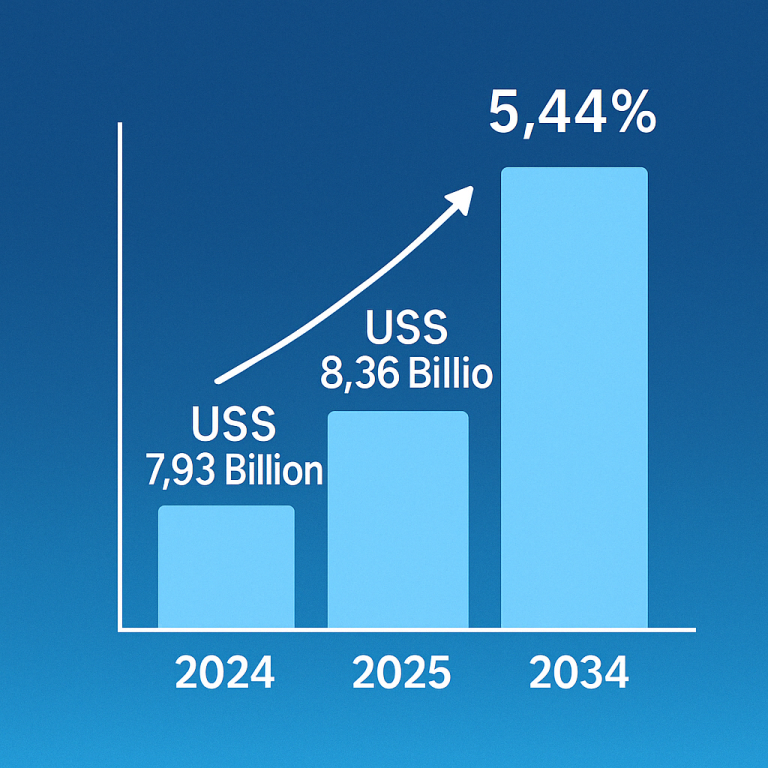
The recent developments in the synovial sarcoma treatment market, spearheaded by Takara Bio, offer newfound hope for patients grappling with this challenging disease. From groundbreaking research to the development of innovative therapies like TBI-1301, Takara Bio’s commitment to advancing the field of oncology is evident. As they continue to navigate the path towards regulatory approval and commercialization, the prospect of more effective treatments for synovial sarcoma becomes increasingly tangible. Through collaboration and dedication, we can collectively strive towards a future where synovial sarcoma is no longer a formidable adversary, but a conquerable challenge.
To Own Our Premium Research Instantly, Click here @ https://www.towardshealthcare.com/price/5121
Unlock Infinite Advantages: Subscribe to Annual Membership
Read More about Synovial Sarcoma Treatment Market: