Advancements in the treatment of ulcerative colitis have been a beacon of hope for millions worldwide. Among these breakthroughs, biological therapies stand out as revolutionary interventions that have reshaped the landscape of ulcerative colitis treatments management. This article delves into the intricacies of biological therapies, exploring their mechanisms, efficacy, and recent developments, particularly focusing on the FDA-approved medication, Velsipty, developed by Pfizer.
Understanding Ulcerative Colitis
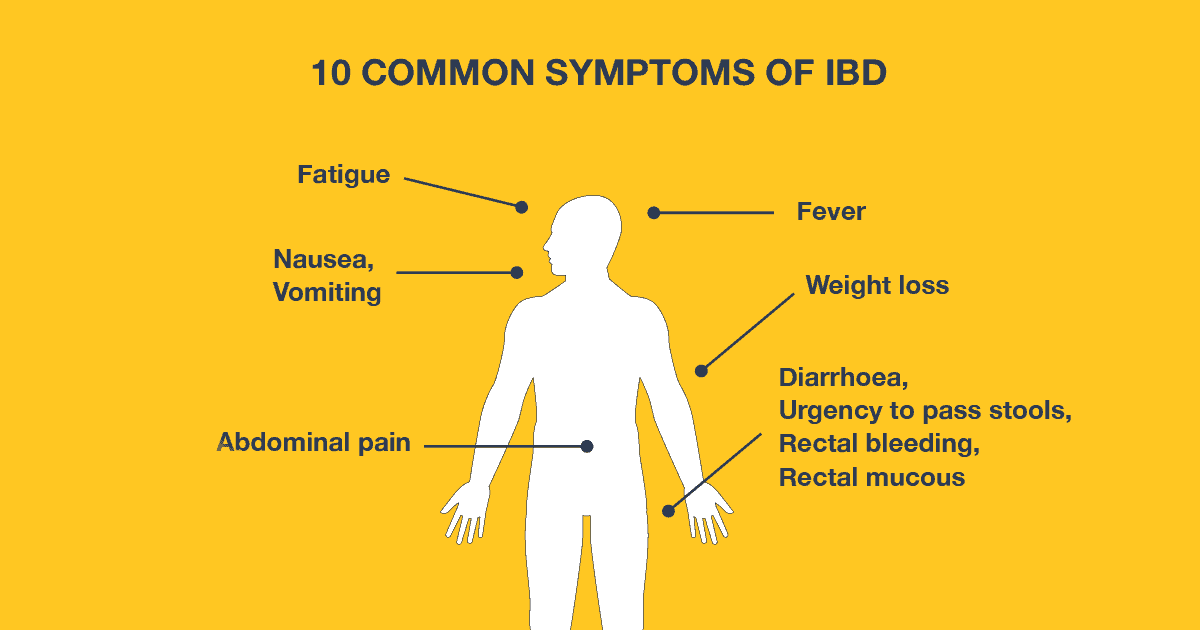
Before delving into biological therapies, it’s crucial to grasp the nature of ulcerative colitis itself. Ulcerative colitis is a chronic inflammatory bowel disease characterized by inflammation and ulcers in the colon and rectum. Symptoms include abdominal pain, diarrhea, rectal bleeding, and weight loss. The exact cause of ulcerative colitis remains unknown, but it is believed to involve a combination of genetic, environmental, and immune system factors.
The Evolution of Treatment Approaches
Historically, the management of ulcerative colitis has relied on a range of medications aimed at reducing inflammation and controlling symptoms. Traditional treatments include aminosalicylates, corticosteroids, immunomodulators, and antibiotics. While these therapies have provided relief for many patients, their efficacy can be variable, and they may come with significant side effects, particularly with prolonged use.
For any queries, we are there to solve them @ https://www.towardshealthcare.com/personalized-scope/5118
The Rise of Biological Therapies
Biological therapies represent a paradigm shift in the treatment of ulcerative colitis. Unlike conventional medications, which broadly suppress the immune system, biologics target specific molecules involved in the inflammatory process. By honing in on these targets, biologics can dampen inflammation more precisely, leading to improved outcomes and reduced side effects.
Mechanism of Action
Biological therapies for ulcerative colitis are typically monoclonal antibodies or fusion proteins designed to inhibit key inflammatory pathways. One example is tumor necrosis factor-alpha (TNF-alpha) inhibitors, which block the action of TNF-alpha, a pro-inflammatory cytokine implicated in ulcerative colitis. Other biologics may target integrins or interleukins, further modulating the immune response and inflammation in the gut.
The Promise of Precision Medicine
One of the most significant advantages of biological therapies is their precision. By selectively targeting specific molecules or cells involved in the disease process, these medications can achieve highly tailored therapeutic effects. This precision not only enhances efficacy but also minimizes collateral damage to healthy tissues, reducing the risk of adverse reactions.
Recent Advances: The FDA Approval of Velsipty
In October 2023, the FDA granted approval for Velsipty, a novel biologic medication developed by Pfizer, to treat severe ulcerative colitis in adults. This approval followed rigorous evaluation in two pivotal clinical trials, ulcerative colitis 52 and ulcerative colitis 12, where participants received a daily 2-milligram dose of Velsipty. The results demonstrated significant improvements in disease activity, with approximately 32% and 26% of participants experiencing remission in the respective trials.
Clinical Efficacy and Safety Profile
The clinical efficacy of Velsipty and other biologics in ulcerative colitis has been well-documented in numerous trials and real-world studies. These medications have shown efficacy in inducing and maintaining remission, reducing the need for corticosteroids, and improving patients’ quality of life. Furthermore, biologics are generally well-tolerated, with adverse events typically being mild to moderate in nature.
Challenges and Considerations
While biological therapies offer promising benefits, their use is not without challenges. One significant consideration is the cost, as biologics tend to be expensive compared to conventional treatments. Access to these medications may also be limited in some healthcare systems, raising concerns about equity and affordability. Additionally, as with any immunosuppressive therapy, there is a potential risk of infections and other adverse effects that must be carefully monitored.
Future Directions and Research Opportunities
Looking ahead, ongoing research aims to further refine and expand the therapeutic options for ulcerative colitis. This includes investigating novel targets, optimizing treatment regimens, and exploring personalized approaches to therapy. Additionally, efforts to enhance patient education and support are crucial for maximizing the benefits of biological therapies and improving long-term outcomes.
Biological therapies have emerged as a cornerstone of modern ulcerative colitis management, offering targeted interventions that hold the promise of improved outcomes and enhanced quality of life for patients. With the recent FDA approval of Velsipty and ongoing advancements in research and development, the future looks increasingly hopeful for individuals living with this challenging condition.
To Own our Premium Research Study in Detail, Click here https://www.towardshealthcare.com/price/5118
Unlock Infinite Advantages: Subscribe to Annual Membership
Read More About Ulcerative Colitis Treatment Sector: