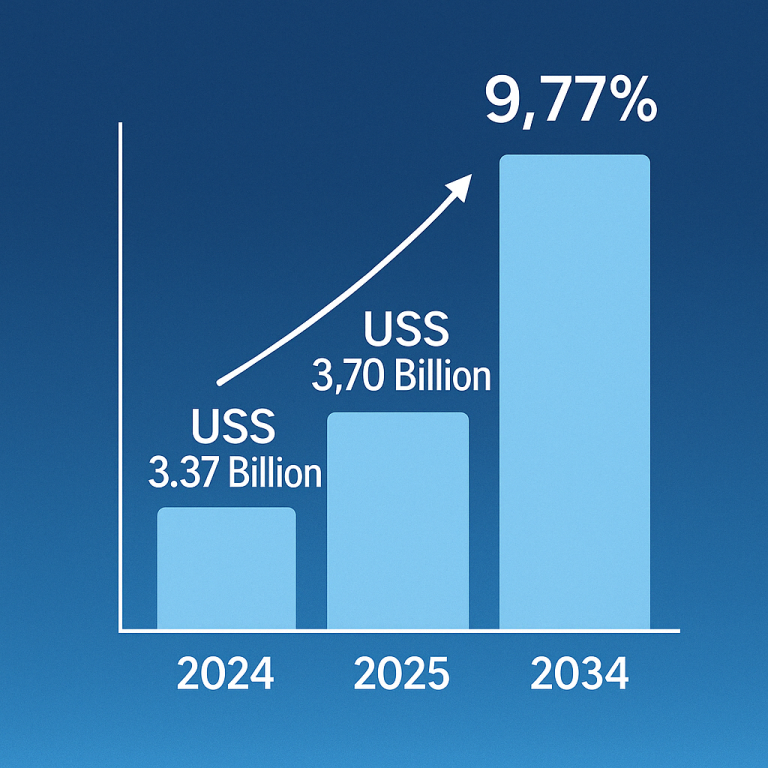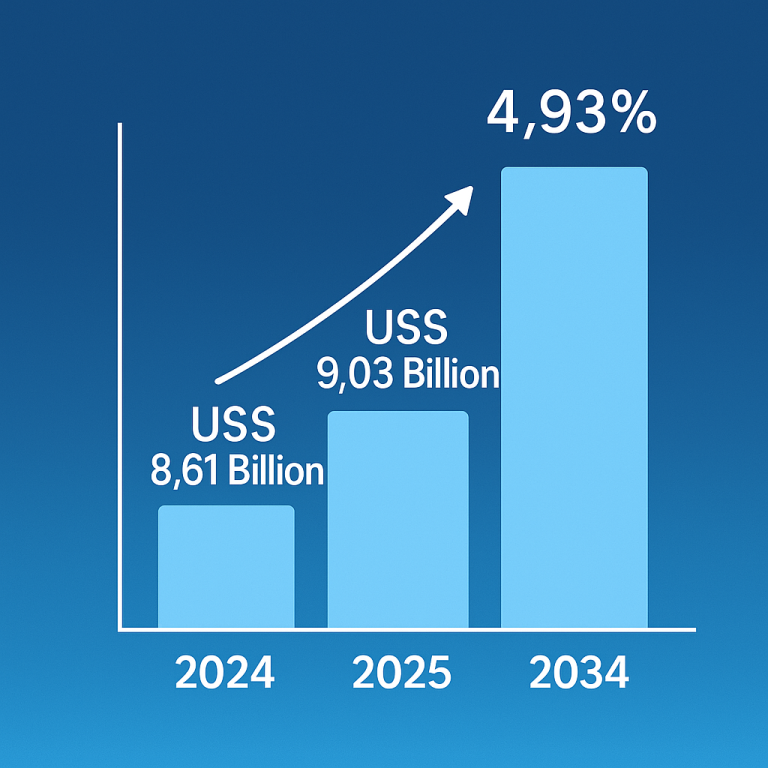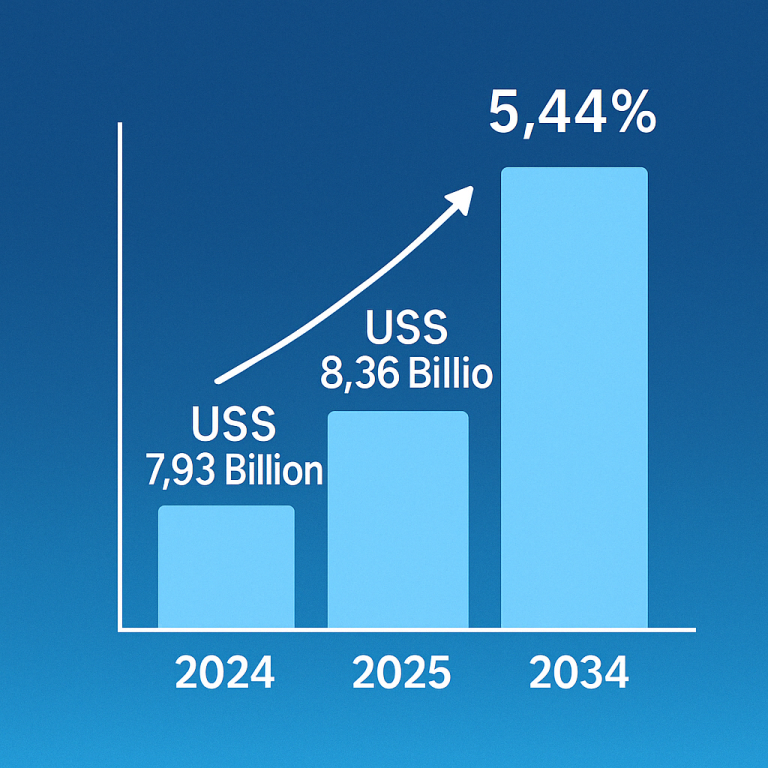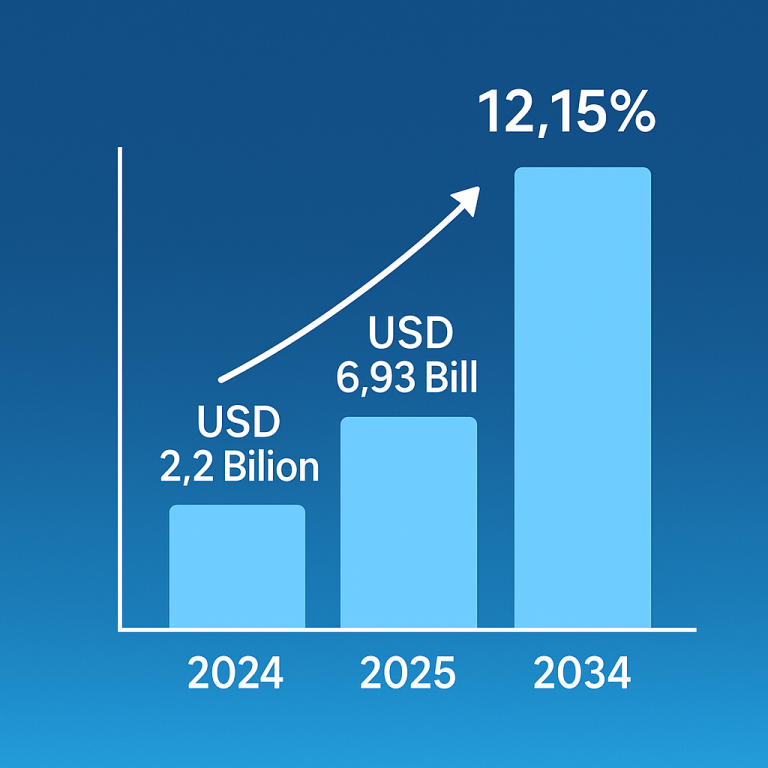
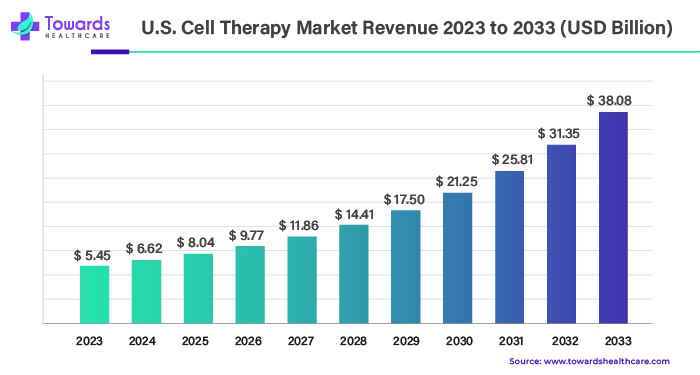
Cell therapy, a cutting-edge medical treatment involving the transplantation of human cells, has been making substantial strides in various medical fields. The U.S. cell therapy market, valued at $5.45 billion in 2023, is projected to reach $38.08 billion by 2033, registering an impressive compound annual growth rate (CAGR) of 21.46% from 2024 to 2033. This growth is being driven by the increasing prevalence of chronic conditions, which is fueling demand for innovative treatments. Among the key drivers of this market expansion are autologous and allogeneic cell therapies, both of which are evolving rapidly and offering promising treatments for a range of diseases, including cardiovascular disorders, cancer, neurodegenerative conditions, and orthopedic issues.
Get All the Details in Our Solution – Download Brochure @ https://www.towardshealthcare.com/download-brochure/5159
Autologous Cell Therapy: Personalized Treatment with Lower Risk
In 2023, the autologous segment dominated the U.S. cell therapy market. Autologous cell therapy involves harvesting cells or tissues from the patient themselves, which are then processed and reinfused to treat a variety of health conditions. This approach includes treatments like autologous skin grafting, autologous CAR T cell therapies, and platelet-rich plasma therapy.
One of the key benefits of autologous therapies is the reduced risk of immunological rejection, as the patient’s own cells are used. This significantly decreases the chances of adverse reactions and improves the likelihood of treatment success. Furthermore, autologous treatments are highly personalized, allowing for targeted interventions and a more tailored approach to patient care.
The versatility of autologous therapies is evident, as they are used to treat an array of conditions, such as cardiovascular diseases, cancer, neurodegenerative disorders, and orthopedic issues. The ongoing advancement of autologous cell treatments continues to unlock new potential for these diseases.
City of Hope’s Landmark Autologous Stem Cell Transplant Surgery
A noteworthy development in the autologous cell therapy space occurred in March 2024 when the Blood and Marrow Transplantation (BMT) and Cell Therapy Program at the City of Hope Cancer Center Atlanta was launched. City of Hope, one of the largest cancer research and treatment organizations in the U.S., has a long history of success in stem cell transplantation. Since its founding in 1976, the organization has performed nearly 19,000 successful transplants.
As part of its expansion into Atlanta, City of Hope will perform its first autologous stem cell transplant surgery this spring, marking a significant step in enhancing patient care and advancing treatment options. The program will also offer BiTE treatment, further improving the availability of cutting-edge therapies for patients.
Allogeneic Cell Therapy: The Growing Alternative
While autologous therapies have led the market in recent years, the allogeneic therapy segment is expected to grow at the fastest compound annual growth rate (CAGR) during the forecast period. Allogeneic cell therapies use cells from a donor rather than the patient’s own cells. This segment is growing due to the variety of cell sources available, including donated tissues, bone marrow, induced pluripotent stem cells, and even umbilical cord blood.
One of the key advantages of allogeneic cell therapy is the availability of diverse cell sources, which can be used for treatment. This variety opens up more options for patients and provides new opportunities for regenerative medicine. For instance, patients suffering from conditions such as Sezary syndrome, myelodysplastic syndrome, acute myeloid leukemia, and cutaneous T cell lymphoma may benefit from allogeneic stem cell transplantation (Allo-SCT).
Mesenchymal Stem Cells and Other Allogeneic Therapies
A growing area of interest within allogeneic cell therapy is the use of mesenchymal stem cells (MSCs). These adult stem cells offer a solution to some of the challenges associated with allogeneic therapies. MSCs can be designed to evade the recipient’s immune system, potentially reducing the risk of rejection and improving the therapy’s effectiveness.
Other forms of allogeneic therapies include hematopoietic stem cell transplantation, induced pluripotent stem cell therapy, and chimeric antigen receptor T cell therapy. These therapies are rapidly being explored for their potential to treat a variety of diseases and improve patient outcomes.
VetStem’s Pioneering Allogeneic Cell Therapy for Canines
In April 2024, VetStem, Inc. made a major move in the allogeneic therapy market with the introduction of the first allogeneic off-the-shelf cell medication product in the U.S. The product, which is a leucoreduced, allogeneic, pooled, freeze-dried platelet-rich plasma (PRP), is intended for intra-articular injection in canines. This breakthrough represents the growing potential of allogeneic cell therapies outside of human medicine. The horse version of this product is also expected to pass FDA scrutiny in May 2024 and enter the market soon after.
Our Table of Content (TOC) covers key healthcare market segments, materials, technologies and trends—helping you navigate market shifts and make informed decisions: https://www.towardshealthcare.com/table-of-content/us-cell-therapy-market-sizing
Invest in Our Premium Strategic Solution @ https://www.towardshealthcare.com/price/5159
We’ve prepared a service to support you. Please feel free to contact us at sales@towardshealthcare.com
Web: https://www.towardshealthcare.com
Visit Dental Specifics: https://www.towardsdental.com
Get the latest insights on industry segmentation with our Annual Membership: Get a Subscription
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare